Combustion (bomb) Calorimeter, Specific heat & calculation of the quantity of heat
Heat and temperature
Heat is a form of energy , The flow of heat from one position to another depends on the difference in temperature between them, The atoms or molecules of substances are in a continuous motion ( vibration ) , but they differ in their speed in the same substance, Temperature is a measurement of the average kinetic energy of matter molecules and it is an indication of hotness and coldness of an object .
When the system absorbs the heat energy , the average speed of its molecules increases, This expresses the kinetic energy of the molecules and leads to the rise of temperature of the system and vice versa ( direct relationship ) , Kinetic energy is expressed by the average speed of molecules not by speed of molecules because of the difference in speed of molecules in one substance .
There are two measuring units of quantity of heat lost or gained by the system :
Calorie is the quantity of heat needed to raise the temperature of 1 g of water by 1 ° C ( 15° C : 16° C ) , Joule is the quantity of heat needed to raise the temperature of 1 g of water by ( 1 ⁄ 4.184 ) ° C .
The Calorie is used when calculating quantity of heat gained from the food , The level of your Calorie consumption depends on the level of your activity .
For example , if you spend a day working in the library , You consume approximately 800 Calories , while a marathon runner consumes approximately 1800 Calories to complete the race .
1 Calorie = 1 kilocalorie = 1000 calorie .
Specific Heat ( Cs )
Specific heat : The quantity of heat required to raise the temperature of one gram of the substance by one degree Celsius ( 1 ° C ) , The unit used in measuring specific heat is J/g . °C .
The specific heat of water is higher than the specific heat of any other substance, The substance that has a large specific heat needs a long time to decrease or increase its temperature – like water – in contrary of the substance that has lower specific heat .
Example : The of heat required to raise the temperature of 1 g of iron by 1° C equals 0.448 J , While the quantity of heat requires to raise the temperature of 1 g of water by 1° C equals 4.18 J .
Specific heat differs according to the type of substance and its physical state , when the specific heat of copper is 0.385 J/g.°C , this means that the quantity of heat required to raise temperature of 1 g of copper by 1°C equals 0.385 J .
Specific heat is a characteristic property for the substance because specific heat is a constant value for the substance , but it differs from one substance to another and also it depends on the physical state of the substance .
Specific heat of water is higher than that of the other substances because the quantity of heat required to raise the temperature of 1 g of water by 1° C is higher than that of other substance.
Water causes a moderate climate in coastal areas in both winter and summer because the specific heat of water is higher than that of the other substances, So, Water can absorb a high quantity of heat in summer and release a high quantity of heat in winter.
In very cold countries, farmers sprinkle water over fruit trees because of the high specific heat of water , thus it protects fruits from freezing.
When two equal masses of copper and aluminum gain the same amount of heat , the temperature of copper ( specific heat = 0.385 J/g.°C ) rises much greater than the temperature of aluminum (specific heat = 0.9 J/g.°C ) because specific heat of copper is less than specific heat of aluminum .
Calculation of the quantity of heat
The quantity of heat ( absorbed or released ) is directly proportional with the difference in the temperature , The quantity of heat needed to raise or decrease the temperature of a system can be calculated by the following relation :
q = m × c × Δ T
q is the quantity of heat measured at constant pressure , m is the mass , c is the specific heat , Δ T is the change in temperature ( Δ T = T2 – T1 ) .
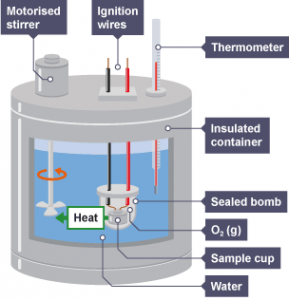
The Calorimeter
It is an isolated system used to determine the change in the temperature of chemical reactions Δ T by knowing each of the initial temperature T1 and the final temperature T2 , It prevents losing or gaining of any quantity of heat or substance with its surrounding .
The Calorimeter is consisted of Isolated container , Stirrer , The reactants ( represent an isolated system ) and Thermometer , There are types of Calorimeters , such as the combustion Calorimeter .
The combustion ( bomb ) Calorimeter
It is used to measure the heat of combustion of some substances , A known amount of substance is burned in an excess amount of oxygen under constant atmospheric pressure, It occurs in an isolated steel container called the steel bomb which is surrounded by an identified amount of the heat exchange liquid ( almost water ) .
The substance is ignited by using an electric wire , The combustion temperature is determined by measuring the change in temperature of the exchange liquid ( water ) , Water is used in the calorimeter as a heat exchange liquid due to its high specific heat which allows it to absorb or lose a large amount of heat energy .
Heat content of a substance ( Molar enthalpy ) and Thermochemical Equation