Nuclear Transformation , Nuclear fission bomb and Nuclear fission reactor
Nuclear Transformation
Nuclear transformation reactions are nuclear reactions in which the nucleus of an element ( called target ) is bombarded with an accelerated particle called projectile ( bomb ) to transform the target into a new nucleus having a new chemical & physical properties , The projectiles ( bombs ) can be accelerated by using devices called nuclear accelerators like The Van de Graaf accelerator & The Cyclotron accelerator .
Using α-particle as a bomb
The first scientist who performed an artificial nuclear reaction was Rutherford in 1919 , where α-particles are used as a bomb and nitrogen gas as a target , Such as Transformation of N-14 into O-17 .
First step : the α-particle ( bomb ) merges with nucleus of the nitrogen atom to form the nucleus of fluorine atom ( unstable ) and is called the compound nucleus .
Second step : The unstable fluorine nucleus gets rid of the excess energy through emitting an accelerated proton and transforms into a nucleus of stable oxygen isotope O-17 within 10−9 sec .
Using proton as a bomb
Example : Transformation of aluminum-27 into magnesium-24
Using deuteron as a bomb
Example : Transformation of magnesium-26 into sodium-24
Using neutron as a bomb
Example : Transformation of lithium-6 into tritium-3+
The neutron is one of the most favorable bombs because it has a neutral charge and it doesn’t meet a repulsion with the electrons surrounding the nucleus , So , it does not require a high energy to enter the nucleus .
Balancing the nuclear equations
During balancing the nuclear equations the following laws must be verified , Charge preservation law and Mass ( Matter ) and energy preservation law .
Charge preservation law
Sum of the reactants atomic numbers ( Z ) = Sum of the products atomic numbers ( Z ) .
Mass ( Matter ) and energy preservation law
Sum of the reactants mass numbers ( A ) = Sum of the products mass numbers ( A ) .
Nuclear Fission Reactions
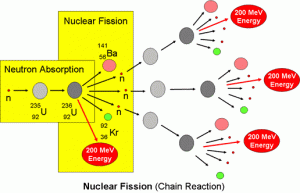
Nuclear fission is the reaction in which a heavy nucleus is bombarded with a light nuclear projectile ( bomb ) of low kinetic energy causing the fission of the heavy nucleus into two nuclei of close masses , a number of neutrons and a huge amount of energy .
Application : Fission of the nucleus of uranium-235
When the nucleus of U-235 atom is bombarded with a neutron , it is transformed into U-236 ( unstable ) that is divided into the two nuclei ( X ) and ( Y ) , which are called the fragments of nuclear fission , in addition to a number of neutrons according to the mass preservation law .
There are 90 variants nuclei that could be produced from this fission , the most common nuclei are barium ( Ba ) and krypton ( Kr ) .
Serial ( chain ) reaction
The neutrons produced from the fission reaction act as new projectiles ( bombs ) that make new similar fission reactions and , So , they split other nuclei of U-235 , So , this reaction is called chain ( serial ) reaction .
Chain reaction is a fission nuclear reaction in which the produced neutrons act as new projectiles ( bombs ) for new nuclei to ensure continuity of the reaction spontaneously as soon as it starts .
This chain reaction generates a huge amount of thermal energy that increases with the continuity of the fission reaction due to increasing the number of the produced neutrons , This is the basic scientific idea of the nuclear fission bomb .
The idea of making the nuclear fission reactor
The nuclear fission reactor is one of the peaceful applications of the chain ( serial ) nuclear fission reaction because it is considered as a source of heat energy that is used to produce the electrical energy by using steam turbines , In the nuclear fission reactor , the nuclear fission reaction is controlled to produce heat or energy .
The main nuclear reaction inside it is the fission reaction of uranium-235 , The amount of uranium used in the reactor is a certain volume which is called the critical volume to ensure the continuity of the chain reaction in the same slow initial rate to produce energy without an explosion .
Critical ( Definite ) volume
It is an amount of uranium-235 in which one neutron – in average – from each reaction can start a new nuclear fission reaction .
The nuclear chain fission should be controlled in the nuclear reactor by absorbing the neutrons through controlling of :
Inserting cadmium rods between the nuclear fuel rods ( U – 235 )
When these rods placed inside the reactor , the chain nuclear reaction begins to slow down as they absorb the neutrons , while on rising them the chain nuclear reaction increases .
The number of the cadmium rods
When the number of cadmium rods increases , the rate of absorbing neutrons increases , So , the rate of the chain nuclear fission reaction decreases .
The idea of making fission bomb
The nuclear fission bomb is one of the unpeaceful applications of fission reactions , In the fission bomb , the amount of uranium-235 is much larger than the critical volume , at which the reaction will continue with an accelerated rate that will lead to an explosion .
In the nuclear fission reactors , the amount of uranium mustn’t be much larger than its critical ( definite ) volume because it needs an amount of uranium enough to generate energy and avoid the explosion , which can occur as a result of increasing the rate of nuclear fission reaction .