The properties of Suspensions and Colloids
Suspensions
It is a heterogeneous mixture in which the diameter of its particles is larger than 1000 nm and it can be distinguished by the naked eye, such as the sand in the water and Chalk’s powder in the water.
Properties of Suspensions
- A heterogeneous mixture .
- The diameter of its particles is larger than 1000 nm .
- The suspended particles precipitate , if it is left for a short time without shaking .
- The suspended particles can be be seen by the naked .
- The suspended particles can be separated by filtration because filter paper ( ultrafiltration membrane ) can hold the suspended particles, while the water passes through it.
Colloids
It is a heterogeneous mixture in which the diameter of its dispersed particles ranges between 1: 1000 nm and it can be distinguished by the electron microscope .
Properties of Colloids
- A heterogeneous mixture ( apparently homogeneous).
- The diameter of the dispersed particles is 1 – 1000 nm .
- The dispersed particles do not precipitate , if they are left for a short time without shaking .
- The dispersed particles can be seen by the electron microscope only.
- The dispersed particles can’t be separated by filtration .
- The shape depends on its concentration : Concentrated colloids appear as milk or clouds , Diluted colloids appear clear .
The colloid is an intermediate case between the solution and the suspension because the diameter of colloid particles is in the range 1: 1000 nm, which is smaller than that of suspension ( > 1000 nm ) and larger than that of solution ( < 1 nm).
How can you distinguish between the colloid and the true solution ?
By allowing a beam of light from a lamp to fall on each one , The colloid scatters the light because the size of the colloid particles is large enough , this phenomenon is known as Tendal’s phenomenon .
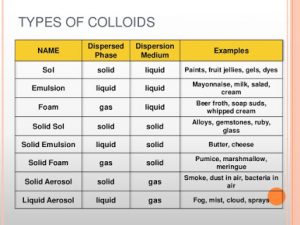
The colloidal systems
They consist of Dispersed phase and Dispersed medium , Dispersed phase ( like the solute in the solution ) , It is the substance that forms the colloidal particles , Dispersed medium ( like the solvent in the solution), It is the medium in which the colloidal particles are dispersed.
Classification of colloidal systems according to the state :
There is no gas-gas colloidal system because mixed gases are homogeneous mixtures , whereas the colloid is a heterogeneous mixture , When an amount of egg white is whipped by an electric mixture , A colloidal system ( gas in liquid type ) is formed .
Preparation methods of Colloids
There are two methods which are Dispersion method and Condensation method .
Dispersion method : The substance is crushed into small particles until its diameter reaches between ( 1 : 1000 nm ) , then added to the dispersed medium with stirring such as starch in hot water and cappuccino coffee .
Condensation method : The small particles are collected together into larger particles have the same volume of the colloid particles , by some processes like Hydrolysis , Oxidation – reduction .
Such as in the reaction of hydrogen sulphide with sulphur dioxide, where the atoms of sulphur in the water form colloid .
Sugar dissolves in the water forming a solution , but milk powder disperses in the water forming a colloid because the size particles of sugar after dissolving is 1 nm, but that in case of milk powder ranges between 1: 1000 nm.
Chalk’s powder forms a suspension in the water because the size of chalk’s particles is > 1000 nm.