The properties of carbon dioxide gas
Carbon dioxide gas is produced from the breathing of humans and animals, It is produced from the combustion of coal or hydrocarbons, the fermentation of liquids, and It is a colorless, tasteless and odorless gas.
Properties of carbon dioxide gas
Carbon dioxide gas (CO2) one of the most stable of all molecules as it consists of a single carbon atom covalently bonded to two atoms of oxygen, and this bond is very strong, and it is a gas at standard temperature and pressure.
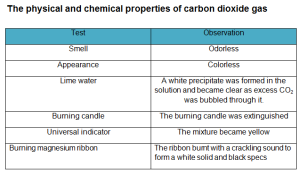
Carbon dioxide gas can change the pH of the water and it dissolves slightly in the water to form a weak acid called carbonic acid, and it is a greenhouse gas as it transmits visible light but it absorbs strongly in the infrared and near-infrared. Carbon dioxide gas does not burn and does not help in burning, so it is used in extinguishing the fires, and it is heavier than the air, so it is collected by upward displacement of air.
Most carbon dioxide units are slightly acidic by nature, the level of acidity can be modified by dissolving the molecules in the water, it is soluble in ethanol and in acetone, It is easily dissolved in the water, so it is not collected by the displacement of water.
Carbon dioxide gas reacts with alkalis to give carbonates and bicarbonates, it is a linear covalent molecule, it is an acidic oxide, and it reacts with the water to give carbonic acid. Carbon dioxide gas reacts with magnesium to form magnesium oxide (white powder) and carbon or coal (black substance) that deposits on the wall of the cylinder.
Carbon dioxide gas is prepared by adding dilute hydrochloric acid to calcium carbonate, it is prepared by upward displacement of air because it is heavier than the air, and it is not collected by displacement of water as it easily dissolves in the water, and it is also prepared by adding lemon juice or vinegar to sodium bicarbonate (the backing powder).
Carbon dioxide gas forms a solid substance at temperatures below -70 degrees Celsius (-94° Fahrenheit), It may also transform into a liquid when it is dissolved in the water under constant pressure, it is very stable, and it is largely unaffected as it interacts with many other materials in the atmosphere.
When we inhale carbon dioxide gas at concentrations much higher than the usual atmospheric levels, it can produce a sour taste in the mouth and a stinging sensation in the nose and throat, as carbon dioxide gas dissolves in the mucous membranes and saliva, and it forms a weak solution of carbonic acid.
Carbon dioxide gas molecule is moderately reactive and is non-flammable, but it will support the combustion of metals such as magnesium, it contains two double bonds and it has a linear shape, It has no electrical dipole, and it is fully oxidized.
Carbon monoxide emissions, sources, effects, uses, poisoning symptoms