The boiling point and separation of the petroleum oil
The boiling point
The boiling point is the temperature at which a matter begins to change from a liquid state to a gaseous state, The change of matter from the liquid state to the gaseous state is known as boiling, and the temperature at which the matter begins to boil is called the boiling point.
The boiling point of the water is 100° Celsius and this means that the water begins to boil and change into the water vapor (gas).
The pressure affects the boiling points, and the boiling points of the liquids vary widely at the normal atmospheric pressure, and because of this variation in the boiling points, two or more liquids can often be separated by a process called the fractional distillation.
At the boiling point, the atoms or the molecules of the liquid gain enough energy to change the liquid into the water vapor, then the boiling is rapidly evaporated.
Life application on the boiling process
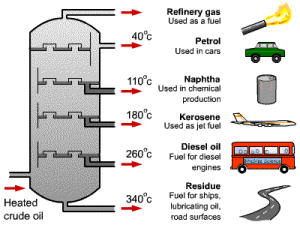
The separation of the petroleum oil depends on the difference between them in their boiling points, The separation of the components of the oil can be done by heating the crude oil, then separating each substance at its boiling point.
The boiling point is the temperature at which the vapour pressure of the substance is equal to the atmospheric pressure, The boiling point depends on the pressure, whereas the pressure increases, the boiling point increases.
The pressure pans are used for fast cooking as they raise the pressure so, the boiling point increases and the food is cooked faster.
Offshore Drilling advantages and disadvantages
Properties of fluids, Factors affecting density and pressure
Applications on the pressure at a point (Connected vessels, U-shaped tube & Mercuric barometer)