Chemical properties of non-metals in modern periodic table
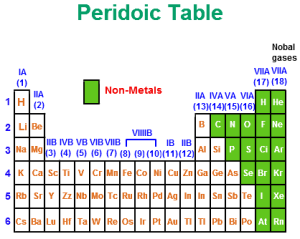
Non-metals
The non-metals are the elements that have more than four electrons in their outermost energy levels, The non-metals are characterized by the smallest atomic sizes and the largest electronegativity.
Sulfur (S) is a nonmetal, It was known as brimstone, Phosphorus (P) is a reactive nonmetal, Graphite is not the only pure from carbon (C), Diamond is also carbon, The colour comes from the impurities caught within the crystal structure.
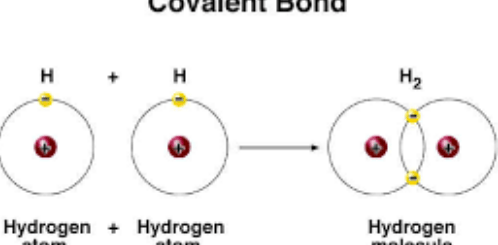
During the chemical reactions, The atom of the nonmetallic elements tends to gain electrons and change into negative ions. The negative ions carry a number of negative charges equal to the number of the gained electrons.
The electronic structure of the negative ions is similar to that of the nearest inert gas that follows the non-metals in the periodic table, The negative ion is an atom of the nonmetallic element gaining an electron or more during the chemical reaction.
During the chemical reactions, the atoms of the elements tend to complete their outermost energy levels with electrons (8 electrons) to reach the stable state, this is done through losing of the electrons as in the case of the metal atoms, and gaining of the electrons as in the case of the non-metals as in the non-metals atoms.
The non-metals (such as carbon and sulphur) don not react with dilute acids (such as HCl), The non-metals such as carbon (C) react with oxygen giving non-metals oxides which are known as “Acidic oxides”. Non-metal acidic oxides dissolve in the water forming acids that turn litmus solution into the red.
You can download Science online application on google play from this link: Science online Apps on Google play