What is the ionic bond? & the characteristics of the ionic compounds
The element is composed of the molecules, Each molecule consists of the atoms, the atom is electrically neutral in its ordinary state (In the atom, the number of the electrons equals the number of the protons, The atoms combine with each other forming the molecules through the chemical bonds.
The ionic bond
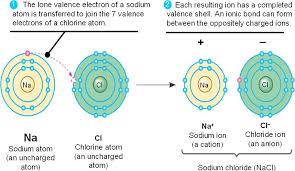
The ionic bond is a type of chemical bonds that occur between a metal atom and a nonmetal atom, The ionic compounds are formed from a metallic cation and a non-metallic anion.
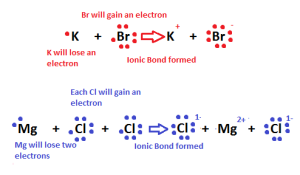
The metal atom loses electrons and changes into a positive ion, the nonmetal atom gains the electrons which lost from the metal atom and changes into a negative ion.
A strong electrostatic attraction between the positive and the negative ions occurs through the ionic bond, The ionic bond is a bond resulting from the electric attraction between a positive ion and a negative ion.
The ionic bonds produce the compounds only not the elements as the ionic bond arises between two different elements (the metal and the nonmetal), The ionic bonds produce the ionic compounds only, and it has one type.
The ionic bond is formed by losing and gaining of the electrons, and it is formed between 2 atoms of two different elements.
The metals with few electrons in its outermost orbital, by losing those electrons, these metals can achieve noble-gas configuration.
Non-metals that have close to 8 electrons in its valences shell tend to accept electrons to achieve noble gas configuration.
The characteristics of the ionic compounds
The ionic compounds are solids at room temperature, they have high melting and boiling points, they dissolve in the water.
They do not conduct the electricity (when they are solids), and they conduct electricity (when they are liquids).