Isoenzymes structure, Coenzymes function and Classification of enzymes
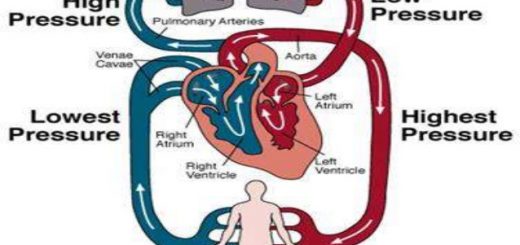
Isoenzymes are alternative forms of the same enzyme activity that exist in different proportions in different tissues, they are known as isozymes, they differ in amino acid composition and sequence and multimeric quaternary structure; but they have similar structures, Their expression in a given tissue is a function of the regulation of the gene for the respective subunits, Isoenzymes are identified in the clinical laboratory by electrophoresis.
Isoenzymes
They are oligomeric enzymes, having the following characters:
- Usually, they have a quaternary structure and the individual subunits in each isoenzyme are different from the others (having different a.a. sequence).
- They act on the same substrate and will give the same product (i.e have the same catalytic activity).
- They have a different affinity to the substrate.
- They are present in different tissues.
- They differ in physical and chemical properties e.g. heat stability, electrophoretic mobility (hence can be separated by electrophoresis), and immunogenic character.
- The direction of reaction differs from tissue to another.
Example: Lactic acid dehydrogenase is a tetrameric enzyme, formed of 4 subunits of two types H and M. Only the tetrameric molecule is active. H and M subunits combined to form 5 isoenzymes as follows:
Isoenzymes of LDH
- H H H H I1 in the heart.
- H H H M I2 and increased in cases of myocardial infarction.
- H H M M I3 in the brain and kidney.
- H M M M I4 in the muscles.
- M M M M I5 in the muscles and liver.
As it was mentioned, isoenzymes of the same enzyme are different in a location in tissues and in their electrophoretic mobility. So, in the case of diseases, we can diagnose the exact site of their release in case of the elevation of the level of the enzyme in the blood by electrophoresis.
Coenzymes
Enzymes may be simple proteins or complex enzymes, containing a non-protein part, called the prosthetic group. The coenzyme is essential for the biological activity of the enzyme. A coenzyme is a low molecular weight organic substance, without which the enzyme cannot exhibit any reaction.
One molecule of the coenzyme is able to convert a large number of substrate molecules with the help of the enzyme. They combine loosely with the enzyme molecules and so, the coenzyme can be separated easily by dialysis. Coenzymes are considered as group carriers and they can be classified:
1. According to the functional basis into:
a. Coenzymes for transfer of H. They are called hydrogen carriers. e.g:
- NAD+ and NADP+
- FMN and FAD
- Lipoic acid
- Coenzyme Q
- Vitamin C
- Glutathione.
b. Coenzymes for group transfer other than H. e.g.
- ATP, GTP, CTP, etc..
- Coenzyme A (CoA)
- Thiamin pyrophosphate (TPP).
- Pyridoxal phosphate (B6)
- Folic acid
- Biotin
2. According to their structural physiological features and according to the origin and chemical structure to:
A. Vitamin coenzymes: coenzyme derivatives of some water-soluble vitamins.
B. Non-vitamin coenzymes:
- Nucleotide coenzymes (UDP-glucose, others nucleotide derivatives of carbohydrates): ATP. Nucleoside: S adenosyl methionine (SAM).
- Peptide coenzymes (glutathione: GSH).
Classification of enzymes
The classification of enzymes is made by the international union of Biochemistry (IUB). There are six major classes: Oxidoreductases, Transferases, Hydrolases, Lyases, Isomerases, and Ligases.
I. Oxidoreductases (redox enzymes):
The oxidation process occurs by 3 means:
Oxidoreductases are classified into oxidases, dehydrogenases, hydroperoxidases, and oxygenases.
A) Oxidases: they are enzymes that catalyze the removal of hydrogen or electrons from the substrate but use only oxygen as a hydrogen acceptor and form water or hydrogen peroxide as a reaction product.
- Oxidases forming water (H2O): e.g. cytochrome oxidase.
- Oxidases forming hydrogen peroxide (H2O2). They are flavoproteins containing either FMN or FAD.
B) Hydroperoxidase: enzymes utilizing H2O2 as a substrate.
H2O2 is injurious to the cells. It is catalyzed by two heme-containing enzymes, catalase, and peroxidases.
Catalase: is present in all cells and tissues, especially abundant in liver, kidney, and erythrocytes.
2 H2O2 → 2 H2O + O2
In the catalase reaction, one molecule of H2O2 act as a substrate, and the other molecule act as a hydrogen donor:
4 HO → 2 H2O + O2
2. Peroxidase present in RBCs, milk, and leucocytes. Peroxidase uses H2O2 as a substrate and an organic substrate as a hydrogen donor. e.g. ascorbic acid, glutathione.
H2O2 + 2GSH → 2 H2O + GS-SG
C) Dehydrogenases: These remove hydrogen from one substrate to a hydrogen carrier. They can not use oxygen as a hydrogen acceptor.
Dehydrogenases are classified according to the type of hydrogen carrier into:
a) Dehydrogenases depend on nicotinamide coenzymes (NAD, NADP)
Lactic acid → Pyruvic acid
b) Dehydrogenases depend on riboflavin coenzymes (FAD and FMN).
Succinic acid → Fumaric acid
d) Oxygenases: They catalyze the direct incorporation of oxygen into the substrate. They are divided into:
- Dioxygenases: Two atoms of oxygen molecules are incorporated into the substrate. As in tryptophan metabolism.
- Mono-oxygenase: Only one atom of molecular oxygen is incorporated into the substrate in the form of the hydroxyl group (termed hydroxylases or mixed-function oxidases), they require a hydrogen donor e.g. NADPH.
Substrate → Substrate + OH
Transferases
These are enzymes which catalyze the transfer of functional groups (G) other than hydrogen between a pair of substrates S and S’.
S-G + S´- G +S
They are divided into the following groups: Transglycosylases, Transphosphorylases, Transcylases, Transaminases, and Transmethylases.
Hydrolases
These enzymes act by splitting (cleavage) of a certain bond by adding water. They are subdivided according to type of bond into Glycosidases, Esterases, endopeptidases, and exopeptidases.
Lyases
These enzymes catalyze the addition or removal of groups e.g. H2O, NH3, or CO2, Without hydrolysis, oxidation or reduction. e.g. Dehydratases (or hydratases): they catalyze removal or addition of water to or from the substrate. e.g.
Fumarase: Malic acid ↔ Fumaric acid + H2O2
Isomerases
They catalyze the interconversion of two isomers. They include the following:
Aldose-ketose isomerases: e.g. Glucose-6-P→ Fructose-6-P
Ligases
These enzymes link two molecules using energy from ATP for example, Carboxylase: (C-C)
Acytyl CoA + CO2 → Malonyl CoA
Factors affecting the rate of enzyme reaction & Importance of enzyme inhibitors
Enzymes meaning, function, examples & Mechanism of enzyme action
Protein Classification, Globular & Fibrous protein, Simple, Compound & Derived proteins
DNA Repair types, definition & importance, Protein Biosynthesis & Genetic Code
Importance of Anatomical body position, planes & terms of movement