The properties of the noble (inert) gases
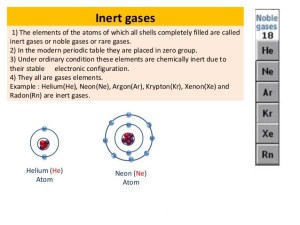
The inert gases are the elements in which the outermost electron shells are completely filled with the electrons, so they don’t participate in any chemical combination in the ordinary conditions.
The noble gases do not form positive or negative ions in the ordinary conditions, The elements of the atoms of which all the shells completely filled are called the inert gases or the noble gases or the rare gases.
Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn) are the inert gases, The noble gases make a group of chemical elements with similar properties under standard conditions.
The noble gases are odorless, colorless, monoatomic gases with very low chemical reactivity, and each molecule consists of one single atom (monoatomic).
The noble gases have weak interatomic force, they have low melting and boiling points, they are all monoatomic gases under the standard conditions, The noble gases are located in the far-right column of the periodic table, they are in group (0) or group (18).
They have a valence of zero, their atoms cannot combine with other elements to form compounds, The noble gases have full valence electron shells, they are stable and do not tend to form chemical bonds.
The noble gases are commonly used in lighting because of their lack of chemical reactivity, and they glow in distinctive colours when they used inside gas-discharge lamps such as neon lights.
Some noble gases have direct application in medicine, and the noble gases are used in the excimer lasers.
Chemical combination, Properties of Metals, Nonmetals & Noble (inert) gases
Classification of elements in Long-form periodic table, Ionization Energy and Oxidation numbers
Radius property, Ionization potential, Electron affinity & Electronegativity
Modern periodic table and classification of Elements
Theories explaining the covalent bond, Octet rule & Overlapped orbitals concept