Calculation of the chemical formula and Types of chemical formulas
The chemical compound is represented by a chemical formula which is a simple symbolic formula that indicates the element percentage and the number of atoms or ions of each element , The chemical compound is made up of units called molecules or formula units which consist of atoms or ions of two or more elements.
Types of chemical formulas
The types of chemical formulas are the empirical formula , The molecular formula and the structural formula .
The empirical formula
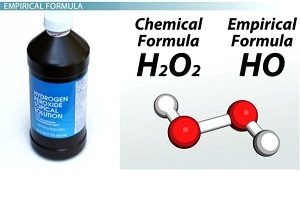
It is a symbolic chemical formula which represent the simplest whole number ratio of the atoms of elements in a compound , The empirical formula does not represent the actual composition of the molecule or the formula unit .
Applications : P2O5 is the empirical formula for P4O10 , CH2O is the empirical formula for CH2O , C2H4O2 , C6H12O6
The molecular formula
It is a symbolic chemical formula which represents the type and the real number of atoms or ions in a molecule or the formula unit of a compound .
In most cases the molecular formula is a multiple of the empirical formula , In some cases the empirical and molecular formulas are identical such as carbon dioxide CO2 , nitric oxide NO and formaldehyde CH2O
In some cases many compounds may have the same empirical formula such as : Acetylene C2H2 and benzene C6H6 have the same empirical formula ( CH ) .
Calculating the percentage of the components of a compound
We use the expression of the mass percentage to calculate the ratio of each component in a certain compound, The mass percentage is the number of units from the particle for each 100 units from the overall .
Calculating the percentage of an element from a chemical formula
% of element = ( The mass of element in one mole of compound ÷ Molar mass of compound ) × 100 %
The following equation shows how the percentage of an element in a compound is calculated
We can calculate the mass of an element in one mole of the compound as follows :
Mass of an element in one mole of the compound = ( Its percentage × Molar mass of the compound ) ÷ 100%
Calculating the percentage of an element from a practical results
The percentage of an element can be calculated from the experimental results as follows :
% of element = ( Mass of element in the sample ÷ total mass of the sample ) × 100 %
The molecular formula and the empirical formula for each of carbon monoxide CO and nitric oxide NO are the same because the molar mass of the empirical formula equal the molar mass of the molecular formula .
In most cases the empirical formula can not represent the chemical structure of a compound because the empirical formula does not represent the actual number of atoms , ions or formula units which form the molecule of the compound .
Both of actylene C2H2 and benzene C6H6 have the same empirical formula CH , but they differ in the molecular formula , They have the same empirical formula because they have the same ratio of elements forming both of them (1: 1 ), But , They differ in the molecular formula because of the difference in their molecular mass and also number of units of the empirical formula .
Theoretical yield , practical yield and percentage of yield
The theoretical yield of the reaction is the calculated quantity of product expected from given quantities of reactants, The practical yield of the reaction is the quantity of product that is actually produced from the chemical reaction .
The percentage of yield = ( practical yield ÷ Theoretical yield ) × 100 %
In most reactions, the practical yield is less than the theoretical yield and the percentage of yield is less than 100 %
Reasons of reducing in yield :
- Some of product may clink-on ( stick ) to the walls of the reactions cylinder .
- The reactants are not pure enough .
- The participation of the reactants in other reactions which are called side reactions .
- The product is volatile ( easily evaporated ) .