What is the melting point? & Life applications on the melting process
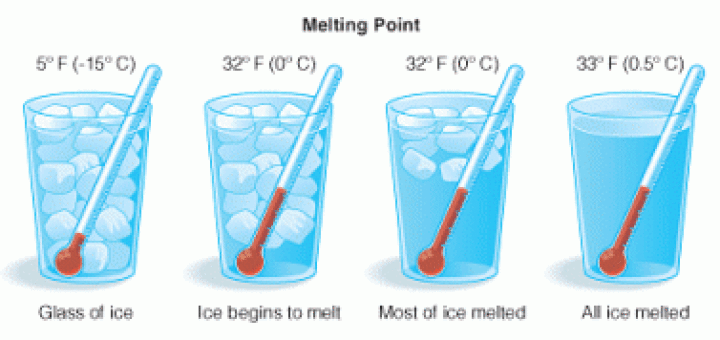
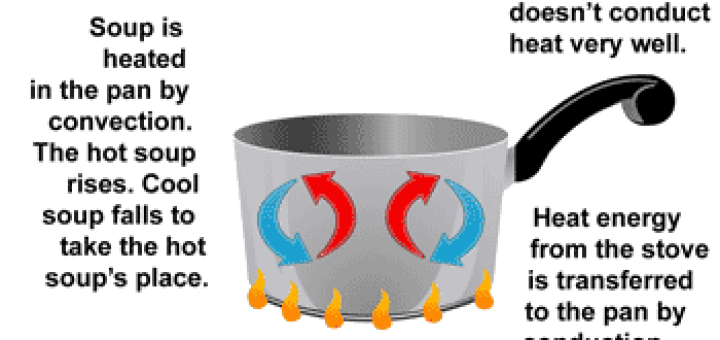
The melting point The matter exists in three states which are solid, liquid and gas, and the matter can change from one state to another by heating, The change of matter from the solid-state...