What is the melting point? & Life applications on the melting process
The melting point
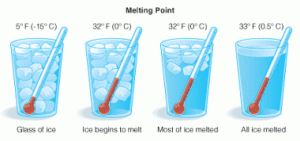
The matter exists in three states which are solid, liquid and gas, and the matter can change from one state to another by heating, The change of matter from the solid-state to the liquid state is known as melting and the temperature at which the matter begins to melt is called the melting point.
The melting point is the temperature at which a matter begins to change from state to a liquid state, The melting point of ice is zero degree celsius where the ice begins to change into the water.
Each substance has a definite melting point which is used to differentiate between different substances, Different solids have different melting points, where some solid substances have low melting points such as wax, butter & ice.
Some solid substances have high melting points such as iron, copper, aluminum and table salt, The melting point of a substance depends on the pressure and it is usually specified at standard pressure.
Measurements of the melting point of a solid can provide information about the purity of the substance where mixtures tend to melt at temperatures below the melting points of the pure solids.
Life applications on the melting process
The workmen melt the solid metals to be easy for mixing and shaping in the manufacture of alloys, Alloys are very important in our life such as copper-gold alloy and nickel-chrome alloy.
Copper-gold alloy is an alloy used in making of jewels which used for decoration, and nickel-chrome alloy is an alloy used in making of heating coils, The materials with high melting points are valuable for making the products which need to resist high heat.
Tungsten has an extremely high melting point, and it is used in the filaments for the light bulbs, If you have a mixture of several solid substances, melting is a way by which some substances (with lower melting points) can be separated from others (with higher melting points).
Matter, Properties & Kinds of molecules, Melting process & Vaporization process
Atomic structure of matter, Energy levels, Electronic distribution & chemical activity

It’s good
Thank you for your comment