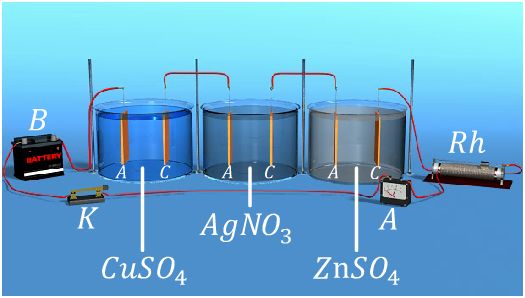
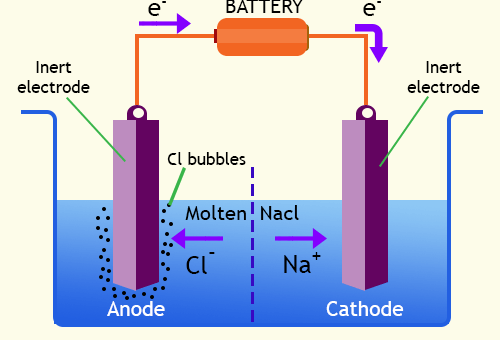
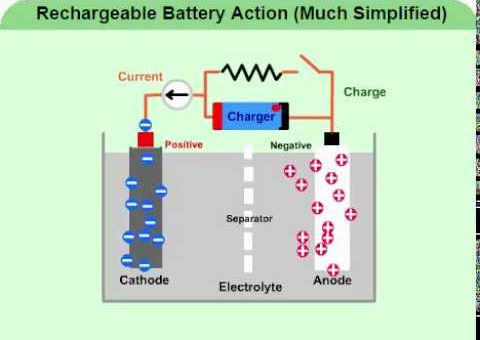
Faraday’s laws of electrolysis (Faraday’s first laws & Second law), Quantity of electricity and equivalent weight
Faraday’s laws are used to calculate the quantity of electricity which flows in solution and quantity of material liberated at electrodes, Faraday deduced the relationship between the quantity of electricity which flows in a...