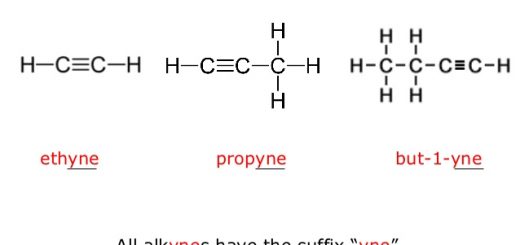
Mechanism of dissolving process and factors affecting solubility
Dissolving process
It is the process that occurs when the solute decomposes into negative and positive ions or into separated polar molecules , Each of them binds to the molecules of the solvent .
Mechanism of dissolving process
Although the water molecules seem static in the beaker, but they are really in a continuous motion, especially the surface molecules ( due to their kinetic energy ).
Dissolving ionic compounds and polar compounds in a polar solvent
It is easy to dissolve ionic compound ( as sodium chloride ) & polar covalent compound ( as hydrogen chloride gas ) .
Dissolving of sodium chloride in the water
The polar water molecules collide with the crystals of NaCl by their kinetic energy , The water molecules attract Na+ and Cl− ions by directing the suitable pole towards them to be separated from NaCl crystals .
The water molecules which surround the ions isolate positive ions from negative ions and prevent their binding again, The speed of the dissolving process depends on the surface area of the solute , the stirring process and the temperature .
Solubility
It is the ability of the solute to dissolve in a certain solvent or it is the ability of the solvent to dissolve a certain solute .
Degree of Solubility is the amount of solute in grams , which dissolves in 100 g of solvent to form a saturated solution at saturated temperature and pressure ( STP ) .
Factors affecting solubility
The solubility is affected by the temperature and the nature of solute & solvent .
The nature of solute & solvent
Like dissolve like is well known statement which controls the solubility process , this statement can be explained as follows :
Polar solvent such as the water dissolves ionic compounds ( sodium chloride NaCl, sodium hydroxide NaOH, Nickel nitrate Ni(NO3)2 ) and the water dissolves polar compounds ( Hydrogen chloride HCl, Ammonia NH3 ).
Non-polar solvent ( organic solvent ) such as Benzene dissolves non-polar compounds ( methane , oil , fats ) and Methane dichloride dissolves non-polar material ( iodine ) .
Solubility of some substances in polar and non-polar solvents
Three tubes contain a heterogeneous mixture of the water and methane dichloride .
In the first tube , the methane dichloride does not dissolve in the water , because the water is a polar solvent, while the methane dichloride is a non-polar substance , in which the non-polar substances do not dissolve in polar solvents .
In the second tube , by adding the iodine solution to that heterogeneous mixture , it dissolves in methane dichloride, but does not dissolve in the water because the iodine solution is a non-polar substance and methane dichloride also is a non-polar solvent , but the water is a polar solvent , In which the non-polar substances do not dissolve in the polar solvents, but dissolve in the non-polar solvents .
In the third tube , By adding green nickel nitrate to that heterogeneous mixture , it dissolves in the water and does not dissolve in methane dichloride because nickel nitrate is a polar substance and the water is a polar solvent , while methane dichloride is a non-polar solvent , In which the ionic substances dissolve in the polar solvents , but do not dissolve in the non-polar solvents .
Oil is insoluble in the water because the water is a polar solvent and oil is a non-polar compound , So, oil does not dissolve in the water .
Oil is soluble in benzene because oil ( non-polar substance ) is dispersed between molecules of benzene ( non-polar solvent ) due to the weak bonds between the benzene molecules .
Sugar is soluble in the water, although sugar is a non-polar substance because the water molecules make hydrogen bonds with the sugar molecules ( polar hydroxyl groups ).
Effect of temperature on solubility
There are three cases , The solubility of most ionic substances increases greatly by increasing the temperature , such as NaNO3 , KNO3 , KCl , KClO3 , The solubility degree of potassium nitrate ( KNO3 ) is 20 g/100 g H2O ( l ) at 10 degree Celsius , On raising the temperature to 50 degree Celsius , the solubility increases and becomes 90 g / 100 g H2O ( l ) .
The solubility of some ionic substances increases slightly by increasing the temperature , such as NaCl , The solubility of some ionic substances decreases by increasing the temperature, such as Ce2(SO4)3 .
Properties of solution
- The particles of solution cannot be distinguished by the naked eye or by the electron microscope .
- The diameter of its particles ( ions or molecules ) is less than 1 nm .
- The particles forming the solution are regularly distributed, So, the solution is homogeneous in its composition and properties.
- The particles do not scatter the beam of light passing through the solution .
Concentration of solutions
The ratio of the amount of the solute to that of the solvent affects the concentration of solution to be diluted or concentrated , Concentrated solution is the solution in which the amount of the solute is large ( not larger than the solvent ) .
Diluted solution is the solution in which the amount of the solute is small in proportion to the amount of the solvent , There are several methods for expressing the concentration which are Percentage , Molarity and Molality .
Percentage
This method is suitable to express concentration for food and medicines .
Mass percentage ( m/m )
Mass percentage is the percentage of the mass of solute in 100 g of solution .
Mass percentage = [ Solute mass ( g ) ÷ Solution mass ( g ) ] × 100 %
Solution mass = Solute mass + Solvent mass
When the mass percentage of a solution is 25 %, the mass of the solute in 100 g of the solution equals 25 g.
Volume percentage ( v/v )
Volume percentage is the percentage of the solute volume in 100 ml of solution .
Volume percentage = [ Solute volume ( ml ) ÷ Solution volume ( ml ) ] × 100 %
Solution volume = Solute volume + Solvent volume
Some solutions are prepared on a volume percentage because liquid volumes are so easily to be measured .
When the volume percentage of a solution is 20 % , The solute volume in 100 ml of the solution equals 20 ml .
The stickers placed on medicines and nutritional substances must show the units that express the solute percentage , due to the presence of many types of percentage .
Molarity ( M )
It is the number of moles of the solute dissolved in one litre of solution , The unit of molarity is ( mol / l ) or Molar ( M ) .
Molarity ( M ) = [ Number of moles of the solute ( mol ) ÷ Solution volume ( L ) ] × 100 %
Number of moles ( n ) = Mass of solute ( g ) ÷ Molar mass ( g/mol )
Molality ( m )
It is the number of moles of solute in one kilogram of solvent , The unit of molality is mol / kg or ( m ) .
Molality ( m ) = Number of solute moles ( mol ) ÷ Mass of solvent ( kg )