Quantum numbers and principles of distributing electrons
The mathematical solution of Schrodinger’s equation introduced four numbers that were called quantum numbers, to determine the energy of an electron in multi-electron atoms, we should know the four quantum numbers which describe it.
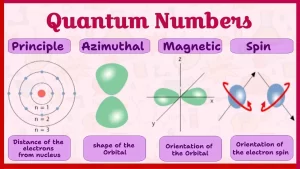
Quantum numbers
These four quantum numbers are:
- The principal quantum number (n): It describes the distance of the electron from the nucleus.
- The subsidiary quantum number (l): It describes the shapes of the electron cloud in the sub-levels.
- The magnetic quantum number (ml): It describes the shape and the number of the orbital in which the electron exists.
- The spin quantum number (ms): It describes the spin of the electron.
The principal quantum number (n)
Bohr had used this number in explaining the spectrum of the hydrogen atom, it is given the symbol (n) and it is used to define the following:
The order of principal energy levels or electron shells, Their number in the heaviest known atom in its ground state is seven.
The number of electrons (e−) required to fill a given energy level which equals the formula 2n² (two times the square of the shell number).
The principal quantum number has whose number values 1, 2, 3, 4, ……. etc, excluding zero, Each value is expressed by an alphabetical letter that represents a principal energy level, Energy levels increase from K to Q, K < L < M < N < O < P < Q.
The rule 2n² isn’t applied to the energy levels higher than the fourth level, because the atom becomes unstable if the number of electrons exceeds 32 electrons in any level.
The subsidiary quantum number (l)
It is given the symbol (l), It determines the number of sub-levels in each principal energy level, Each principal energy level consists of a number of energy sub-levels equal to its principal quantum number.
The energy sub-levels take the symbols and values, Subsidiary quantum number (l) values [0 : (n − 1)]: Symbols of sub-levels, s = 0, p = 1, d = 2, f = 3)
There is a small difference in the energy of the sub-levels, They can be arranged according to increasing their energy, s < p < d < f.
Examples: What are the probable ( l ) values when n = 3?
Each principal energy level consists of a number of sub-levels which equals its numerical value, the Number of sub-levels = 3.
The probable (l) values range between [0 : (n − 1) ] = [0 : (3 − 1)] = 0, 1, 2
Magnetic quantum number (ml)
It determines the number of orbitals within a certain energy sub-level from the relation (2l + 1).
It determines the spatial orientation (orientation in space) of orbitals.
It is represented by the whole number values (odd) ranging between ( − l, ……., 0, …….., + l).
The orbitals of the same sub-levels are equal in energy, but differ in direction and shape in space, Any orbital can’t be occupied by more than two electrons.
The p-sublevel is completely filled with 6 e−, while the d-sublevel is completely filled with 10 e−, Because the p-sublevel contains 3 orbitals, while the d-sublevel contains 5 orbitals and each orbital is filled with 2 e−.
Example: What are the probable (ml) values when (l = 2)?
The probable (ml) values range between −l, ……, 0, …….., + l
The probable (ml) values: −2, −l, 0, +1, +2
Example: Which of the following probabilities of the quantum numbers of a certain electron includes a mistake?
- n = 3 , l = 2 , ml = − l
- n = 4 , l = 3 , ml = − 2
- n = 1 , l = 1 , ml = + l
The choice (3), because when ( n = 1 ) the probable l and ml values are ( 0 ).
The spin quantum number (ms)
Since any orbital can not be occupied by more than two electrons, each electron spins on its axis during its orbit around the nucleus as the spinning of the Earth on its axis during its rotation around the Sun.
The spin quantum number determines the type of spin motion of the electron around its axis in the orbital, which is either :
- Clock wise (↑) with ms value equals to (+ ½).
- Anticlockwise (↓) with ms value equals to (− ½).
The spin motion of the two electrons of the same orbital around their axis arises a magnetic field in two opposite directions (spin-paired state) (↑↓).
Orbitals have three different possibilities depending on the number of electrons located in them as follows :
- ( ) Empty orbital.
- (↑) Half-filled orbital contains one electron.
- (↑↓) full-filled orbital contains 2 pairing electrons that have opposite spins and called spin paired state.
Although the two electrons of the same orbital carry the same negative charge, they do not repel with each other, because the magnetic field arises from the spinning of one electron is in a direction opposing the direction of the other magnetic field arising from spinning of the other electron, and that decreases the repulsive force between the two electrons.
Summary of the relationship between the principal energy level, sub-levels, and orbitals.
Each principal energy level:
- Consists of a number of sublevels equals its number (n = no. of l values).
- Consists of a number of orbitals equals the number of the level (n²).
- The number of electrons occupying it, equals two times the square of the level number (2n²).
Each sublevel consists of a number of orbitals which equals (2l + 1).
Each orbital is occupied by 2 electrons: (s = 2, p= 6, d = 10, f = 14).
Principles of distributing electrons
There are some important rules, which must be considered in distributing electrons in the atom, These rules are:
- Pauli’s exclusion principle.
- Aufbau (building-up) principle.
- Hund’s rule.
Pauli’s exclusion principle
Pauli’s exclusion principle: It is impossible for two electrons in the same atom to have the same four quantum numbers.
When (np) sublevel contains one electron, it is not necessary to be :
(ml = − l) , but it may be equal to 0 or + l
(ms = + ½), but it may be equal − ½
Aufbau ( building-up ) principle
Aufbau (building-up) principle: Electrons occupy the sub-levels in the order of increasing their energy, and the lowest energy sub-levels are filled first.
The sub-levels of the same principal energy level differ slightly from each other in energy.
The arrangement of sub-levels according to their energy depends on:
The sum of (n + l), Ex: Energy of 4s sub-level is lower than that of 3d sub-level because the sum of (n + l) of 4s sub-level is less that of 3d sub-level.
Order of the principal energy level, in case of equality of sum (n + l) values for two sub-levels, Ex: Energy of 3p sub-level is lower than that of 4s sub-level, Because (n) value of 3p sub-level is lower than that of 4s sub-level.
The sequence of energy sub-levels is arranged ascendingly according to their energy follows
The order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5d < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Filling the energy sublevels: s up to 2 electrons, p up to 6 electrons, d up to 10 electrons, f up to 14 electrons.
The electron configuration for one of the energy levels can be expressed as the following: 2p6
Where 2 is the principal energy level (n), p is the energy sub-level, and 6 is the number of electrons in the orbitals of the sub-level.
Example: write the electron configuration for the following elements, according to the building-up principle: 11Na, 20Ca, 30Zn, 19K, 26Fe.
11Na: 1s², 2s², 2p6, 3s1
20Ca: 1s², 2s², 2p6, 3s², 3p6, 4s²
30Zn: 1s², 2s², 2p6, 3s², 3p6, 4s², 3d10
19K: 1s², 2s², 2p6, 3s², 3p6, 4s1
26Fe: 1s², 2s², 2p6, 3s², 3p6, 4s², 3d6
Hund’s rule
Hund’s rule: No electron pairing takes place in a given sub-level until each orbital contains one electron.
Rules of filling the energy sub-levels with electrons, according to Hund’s rule:
- The orbitals of the same sub-levels are equal in their energy.
- The orbitals of the same sub-levels are filled by the unpaired electrons first, The spin of a single electron in the same direction gives the atom stability.
- When the two electrons are paired in one orbital, they have opposite spins, to decrease the repulsive force between them, then the two electrons are in a spin-paired.
- Electrons pairing takes place in the orbitals of the same sub-level after occupying all orbitals by unpaired electrons.
- The electrons prefer to be paired with another electron in one orbital of the same sub-level rather than being transferred to a higher energy sub-level.
The spin of single electrons in the same sub-levels orbitals is in the same direction because this state gives the atom more stability.
The electron prefers to occupy an orbital alone in the same sub-level rather than pairing with another one in the same orbital, Because when two electrons are paired in one orbital, (in spite of their opposite spins), there must be a repulsive force that decreases the stability of the atom (increasing in its energy).
The electron prefers pairing with another one in orbital in the same sub-level rather than transferring to the higher energy sub-level, because the required energy to overcome the repulsive force between the two paired electrons is less than that is required for transferring the electron to a higher sub-level.
Example: Predict the atomic number for each of the following elements:
An element whose electronic configuration is 1s² , 2s² , 2p3 → 7
An element whose principal energy level ( n = 3 ) contains 3 electrons → The electronic configuration: 1s², 2s², 2p6, 3s², 3p1, So, the atomic number = 13
An element whose last 3s-sublevel is half-filled with electrons → The electronic configuration: 1s², 2s², 2p6, 3s1, So, the atomic number = 11.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science Online application on Google Play from this link: Science Online Apps on Google Play
Atoms components, Rutherford and Bohr’s Atomic Models
Atomic emission spectra, Bohr’s atomic theory, and Wave mechanical theory of the atom