Immunogens, antigens, antibodies, Immunoglobulin Classes and Protective Functions of Antibodies
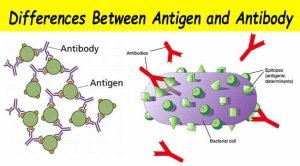
An immunogen is any foreign substance that when introduced into the body stimulates a specific immune response in the form of antibody formation or cell-mediated immunity. For a substance to be immunogenic, it must be recognized by the body as being foreign or non-self. An immunogen is usually a protein or polysaccharide of a higher molecular weight. An antigen is a molecule that can bind to the components of the immune system, including antibodies, T and B cell receptors.
Antigenic determinants (Epitopes)
Epitopes are the chemical features on the antigen molecule that physically bind to antibody (on B cells) or T cell receptors. An antigen can have one or more epitopes.
Examples of some Ags include:
Haptens
A hapten is a protein-free chemical substance, which is incapable by itself of producing an Ab, but when it binds to the body protein, it becomes immunogenic and stimulates Ab production. Haptens are like penicillin, aspirin, sulfa, cosmetics, tranquillizers and formaldehyde. A hapten can react to specific Ab, so when it is introduced into the body for the second time, it will react with the previously formed Ab leading to a reaction.
Iso-antigens (Human tissue antigens)
These are Ags present in the cells of the body of different members of the same species.
Example: is the blood group Ags. There are 2 main systems: the “ABO or ABH” and the “Rh” factor.
Human leucocytic antigen (HLA) is another example of an iso-antigen present in all nucleated tissue cells mainly leucocytes. HLA are of vital importance in organ transplantation.
Auto-antigens
Normally an individual does not react with his organs (self Ags). But, due to infection or inflammation, the antigenic structure will change (recognized as non-self), resulting in the production of autoantibodies as in some autoimmune diseases.
Heterophile antigens
Ags shared by different species, e.g. Epstein-Barr virus (EBV) shares Ags with sheep and horse RBC’s. The antibodies formed in infectious mononucleosis (caused by EBV) agglutinate sheep or horse blood cells in the monospot test.
Superantigens
These are a group of molecules that are active in a very low concentration. They can activate a large number of T-cells non-specifically. They are mostly of bacterial origin and include the staphylococcal enterotoxin (responsible for some types of acute food poisoning), toxic shock syndrome toxin, exfoliative dermatitis toxin and streptococcal pyrogenic exotoxin.
However, some viral proteins as well as some antigens of certain mycoplasma species act as superantigens. They are termed superantigens because they bind directly, i.e, unprocessed to the variable regions of ß-chains (VB chains) of antigen receptors on certain subsets of T-cells and cross-link them to the major histocompatibility complex MHC class II of antigen-presenting cells (APCS) ie, act as a clamp between the two, providing a signal for T helper activation.
The adverse effect of superantigens is the systemic toxicity of the massive cytokine/ lymphokine release of IL 1, IL2 and TNF that results from the simultaneous stimulation of a large subset of T-cells.
Antibodies
Antibodies or immunoglobulins are glycoproteins expressed as:
- Soluble molecules present in serum and tissue fluids, or
- Membrane-bound receptors on the surface of B cells.
Contact between the B cell receptor and the antigen it recognizes results in B cell activation and differentiation to generate plasma cells that secrete large amounts of antibody, The secreted antibody has the same binding specificity as the original B cell receptor.
Immunoglobulin Classes
IgG: It consists of four polypeptide chains, 2 H and 2 L held by disulphide bridges. It is bivalent and it forms 75% of all lgs in the serum. – It is the only one that crosses the placenta. It fixes the complement. There are four subclasses (IgG1 to IgG4), based on antigenic differences in the H-chains and on the number and location of the disulphide bonds.
IgG is the predominant Ab in the secondary immune response.
IgM: It consists of 5 units joined together by the j-chain each unit is similar in structure to one IgG (2 L and 2 H chains), so, IgM is formed of 10 L and 10 H chains, It has the highest molecular weight of lgs (pentamer). IgM comprises about 7% of lgs in normal human serum. IgM is the Ig that appears early in the specific immune response. It fixes the complement and is a good agglutinating Ab.
IgA: The basic structural unit of IgA is similar to that of IgG, but IgA may also be found as a dimer, The monomeric IgA is found in serum, forming 15% of the circulating lgs. The dimers are found in body secretions milk, colostrum, tears, saliva and other gastrointestinal secretions, nasal and bronchial secretions, genital secretions, and urine.
Secretory IgA has a secretory component and a “J” chain. Secretory IgA protects the mucous membranes from attack by bacteria and viruses. There are two subclasses for IgA. IgA1, and IgA2. Some bacteria (Neisseria spp) can destroy IgA by producing (protease and can thus overcome antibody-mediated resistance on mucosal surfaces.
IgD: Found in a small concentration in serum. IgD is found mainly on the surface of B-lymphocytes. These B cells contain IgD and IgM at a ratio of 3 to 1. The function of IgD is unclear.
IgE: Is also called cytotropic Ab. It is found in trace amounts in normal serum. The level of IgE in the serum of allergic individuals is high. It plays an important role in type I hypersensitivity. IgE level in serum is increased very much in parasitic infections.
Immunoglobulin class switching
Initially, all B cells bound to an antigen carry IgM specific for that antigen and produce IgM in response to this antigen. Later, gene rearrangements generate antibodies of the same antigen specificity but of different immunoglobulin classes, so that the immunoglobulin produced later (IgG, IgA or IgE) has the same specificity as the original IgM but with different biologic characteristics, Class switching is dependent on cytokines released from T cells.
Rate of Antibody Production
1. Primary response
if an antigen is injected for the first time in an animal, 7-12 days will lapse before Abs can be detected in the serum, then the level of Abs will rise slowly to a low peak, then it fails. The Ab in the primary response is mainly of the IgM class.
2. Secondary response
If another injection of the same Ag is given the level of Abs will immediately fall a little, due to neutralization of the already present Ab, then it will rise to a higher level (higher peak) and stays longer than the first time. This is due to the presence of immunological memory, If a third injection is given, the same response will occur, but with a higher peak which also stays longer than the second.
This can occur with additional injections, till such a time where there is no further increase in the level of Abs, this is the hyperimmune state, and is needed for effective vaccination, The Ab in the secondary immune response is mainly IgG.
Protective Functions of Antibodies
Antibodies provide protection against extracellular pathogens in the blood, mucosal surfaces and tissues. The protective role is carried out by the following methods:
Precipitation: When antibodies combine with soluble antigen forming a lattice.
Agglutination: When the interaction between antibodies and antigens in a particulate form result in visible clumping.
Virus neutralization: Antibodies bind to the virus and block the ability of the virus particle to attach to its cellular receptor. Because the virus can no longer invade the cell, it cannot replicate.
Neutralization of toxins: Antibodies can neutralize toxins of microorganisms (e.g. tetanus and botulism) and inactivate their harmful effects.
Opsonization (Enhanced phagocytosis): Antibodies by opsonizing (coating) organisms, make them more readily ingested by phagocytes. This engulfment leads to pathogen destruction.
Activation of the complement system: The attachment of antibodies to viral proteins on virus-infected cells, tumor cells, or to a microbial cell can activate the complement system leading to cell lysis.
Antibody-dependent cell-mediated cytotoxicity (ADCC): The lytic destruction of infected or tumor cells is mediated by a killer cell (NK, macrophage, neutrophil, eosinophil) that binds to the Fc portion of the bound antibody.
Monoclonal Antibodies
These are highly specific antibodies produced against a single epitope. They are obtained by fusion of a myeloma cell (malignant plasma cell) with a B cell-producing antibody against a single epitope (derived from the spleen of mice immunized with this epitope).
The resulting fused cell is called (a hybridoma cell), this cell has the ability to produce unlimited quantities of highly specific monoclonal antibodies. This is the murine type. Another humanized one is produced by genetic engineering (recombinant DNA technology). Monoclonal antibodies have several diagnostic and therapeutic uses in the medical field.
Diagnostic use, as in lymphocyte subsets determination, HLA typing (by flow cytometry) and serological tests as ELISA, chemiluminescence and immunofluorescence. Therapeutic as anti-tumour therapy, antiallergic, and immunosuppressive therapy to prevent graft rejection.
You can download Science online application on Google Play from this link: Science online Apps on Google Play
You can follow science online on YouTube from this link: Science online
Classification of medically important Gram-negative bacilli
Control of Drug Resistance & Bacterial resistance to chemotherapeutic agents
Staphylococci definition, diagnosis of staphylococcal infections & treatment
Antibiotics advantages, disadvantages, resistance & uses
Features and classification of viruses, Defective viruses & Viral vectors used for gene therapy