Vaccines types, Live vaccines, Inactivated vaccines, Subunit vaccines, Naked DNA and mRNA vaccines
The immune response activation is faster and stronger in immunized individuals than in unimmunized ones. Immunization of a population can stop the spread of the infectious agent by reducing the number of susceptible hosts (herd immunity) and hence it protects the recipient and the general population. The main property to make a virus candidate for vaccine development is to be the cause of a significant illness.
Vaccines
Immunization programs have achieved:
- Protection of the population from the symptoms e.g rabies and tetanus vaccines.
- Protection and control of the spread of measles, mumps, rubella, varicella zoster, influenza, and rotavirus.
- Elimination of wild-type poliomyelitis in most of the world and smallpox worldwide.
- Reduction in cancer risk caused by the human papilloma virus and HBV.
- N.B. Vaccine-preventable diseases still occur, and outbreaks take place wherever immunization is unavailable due to high expenses (developing countries).
Types of vaccines
- Live vaccines (LAV)
- Inactivated vaccines
- Component Vaccines (subunit vaccine)
- Toxoid vaccines
- Naked DNA vaccines
- mRNA vaccines
1. Live Vaccines (LV)
Live vaccines (LV) are derived from an organism (virus or bacteria) that have been laboratory modified and weakened, so that it can replicate in the host and stimulate immune responses without causing any serious disease. This would resemble the natural infection in stimulating both the natural innate and antigen-specific immune pathways so that humoral, cellular, and memory immune responses are developed.
Examples of LAV
- Viral LAV, as the oral polio vaccine, measles, cold-adapted influenza virus vaccine, and rotavirus.
- Bacterial LAV, as Tuberculosis (BCG) vaccine.
Preparation:
Live vaccines are prepared with avirulent, or attenuated organisms limited in their ability to cause pathology, this may consist of any of the following:
Live Attenuated viral vaccines. Less virulent mutants of the wild-type virus.
- Wild-type viruses are attenuated by growth in animals or tissue culture cells at non-physiologic conditions, attenuation occurs due to unfavourable conditions that select or allows the growth of viral strains that are less virulent e.g.: viruses that grow poorly at 37°c e.g., measles, cold-adapted strains of influenza virus.
- Serial passage in tissue cell lines to induce the ability of the virus to replicate at the benign site without disease development e.g., polio vaccine replicate in GIT but does not reach neurons.
live vaccines attenuated by genetic engineering mutations that inactivate or delete a virulence gene.
Avirulent viral vector vaccine: Recombinant techniques are used to insert gene coding for the protein of interest into the genome of an avirulent vector virus from other species (e.g., vaccinia virus, adenovirus). On infection, it simply promotes the expression of the inserted gene to initiate an immune response.
Advantages:
- The immunity conferred is more efficient and long-lasting.
- The immune response to live vaccines simulates that of natural infection.
- Production of 1g A at the portal of entry.
- Induce a good cell-mediated response.
Disadvantages:
- Theoretical existence of the risk of reversion of the virus to virulence.
- Care for the storage temperature and limited shelf life is a demanding problem, the use of viral stabilizers e.g., mgcl for the polio vaccine helps overcome the problem.
- Interference in case of co-infection with a naturally occurring virus replicating the same host cell.
- It cannot be given to immunocompromised patients or pregnant females.
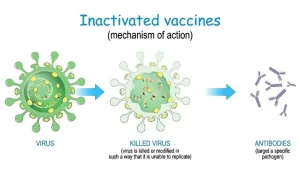
2. Inactivated Vaccines
- Utilize organisms that have been killed so they can no longer replicate in the host.
- Used to elicit protective antibody immune response without the risk of infection.
Preparation
- Usually prepared by exposure of a wild-type (virulent) organism to a chemical agent e.g. formalin, or physical agent as irradiation or heat inactivation, followed by the use of various purification and concentration techniques.
- Adjuvants are added to boost the immunogenicity, as they enhance uptake by presenting cells e.g. aluminium hydroxide or aluminium phosphate.
- Examples of killed (inactivated) viral vaccines: Rabies, Influenza, Polio (Salk), and Hepatitis A.
- Examples of killed (inactivated) bacterial vaccines: Cholera and Q fever vaccines.
Advantages of Inactivated Vaccines
- No risk of reversion to virulence.
- Can be given safely in immunocompromised patients and during pregnancy.
Disadvantages of Inactivated Vaccines
- Immunity is usually brief and not usually lifelong.
- Immunity may be only humoral with satisfactory levels of circulating IgG and IgM while cell-mediated immunity is generally poor.
- The vaccine does not elicit a local IgA response at the portal of entry.
- Booster shots are required to maintain effectiveness.
- Larger doses must be used.
- Some inactivated vaccines can induce hypersensitivity to subsequent infection.
3. Component Vaccines (subunit vaccine)
Subunit vaccines are similar to inactivated vaccines in not containing live components of the pathogen, while they have the advantage over them by containing only the antigenic parts (immunodominant protein) of the pathogen which can elicit a protective immune response. Subunit vaccines can be further categorized into:
- Protein-based subunit vaccines.
- Polysaccharide vaccines.
- Conjugate subunit vaccines.
a. Protein-based subunit vaccines
Examples of Commonly used protein-based subunit vaccines are the following:
- Acellular pertussis (aP) vaccines contain inactivated pertussis toxin (protein) and may contain one or more other bacterial components.
- Hepatitis B vaccines are composed of the hepatitis B virus surface antigen (HBsAg), a protein produced by hepatitis B virus. Currently produced by genetic engineering using recombination technology (expression of cloned viral genes; through Insertion of DNA encoding the immunizing antigen in yeast cells), The antigen would be expressed by the yeast cell, then purified to be used as the immunogen in the vaccine e.g. recombinant Hepatitis B vaccine.
b. Capsular Polysaccharide vaccines
Elicit a response against the pathogen’s capsule which results in anti-capsular antibodies that block the binding of the organism to the mucosal surfaces. E.g: vaccines against Streptococcus pneumoniae, Hemophilus influenzae, Neisseria meningitidis. These polysaccharide molecules are small, and often not very immunogenic. As a consequence, they tend to:
Not effective in infants and young children (under 18-24 months).
2. Induce only short-term immunity (slow immune response, slow rise of antibody levels, produce mainly IgM, no immune memory) because of the inability of polysaccharides to activate T helper cells. So, these vaccines are best conjugated to a carrier protein to enhance the antibody response.
c. Conjugate subunit vaccines
Conjugate subunit vaccines also create a response against the molecules in the pathogen’s capsule, In comparison to plain polysaccharide vaccines, they benefit from a technology that binds the capsular polysaccharide to a carrier protein that can induce a long-term protective response even in infants. E.g., Hemophilus influenzae type b (Hib) and pneumococcal conjugate vaccines.
4. Toxoid vaccines
- A toxoid is an inactivated toxin that has lost its ability to cause disease but has retained its immunogenicity.
- The toxoid is used to stimulate the immune system to produce antitoxins (antibodies) that block the binding of the native toxin to cells.
- E.g. tetanus toxoid.
5. Naked DNA Vaccines
- Vaccines are based on inserting an immunizing gene in naked plasmids without any protein coat.
- It is parenterally injected into the target cells of the host.
- Cells taking the plasmid will express the immunizing protein to stimulate a specific immune response.
6. mRNA vaccines
It uses a molecular-engineered mRNA sequence with a nanoparticle coat.
This mRNA codes for the immunizing specific viral protein.
Once introduced in a host cell, it will be transcribed, and a specific target protein will be expressed on the cell surface.
This subsequently will stimulate a specific cellular immune response, e.g: Covid 19 mRNA vaccine expressing viral spike protein.
You can follow science online on YouTube from this link: Science online
You can download Science online application on Google Play from this link: Science online Apps on Google Play
Adaptive (Acquired immunity) types, Difference between Innate & Adaptive Immune responses
Immune response to infectious agents, Differences between natural & acquired immunity
Features and classification of viruses, Defective viruses & Viral vectors used for gene therapy
Cytokines function, use, definition, inflammation & side effects