Oxidation and Reduction reactions by losing and gaining the electrons
Oxidation and Reduction processes take place by two ways, Losing and gaining oxygen or hydrogen, Losing and gaining electrons, The two processes of oxidation and reduction are concurrent processes as they occur at the same time.
Oxidation & reduction
H2 + CuO Cu + H2O
What happens during the reaction of hydrogen gas passes and hot copper oxide?
Hydrogen is oxidized because it combines with oxygen, Hydrogen is considered as a reducing agent because it took oxygen from copper oxide, Copper oxide is reduced because it loses oxygen, Copper oxide is considered as an oxidizing agent because it gives oxygen to hydrogen.
Oxidation is the chemical process that causes the increase in the oxygen percentage or the decrease in the hydrogen percentage in the substance.
The reduction is the chemical process that causes the decrease in the oxygen percentage or the increase in the hydrogen percentage in the substance.
The oxidation agent is the substance that gives oxygen or takes hydrogen away during a chemical reaction, The reducing agent is the substance that takes oxygen away or gives hydrogen during a chemical reaction.
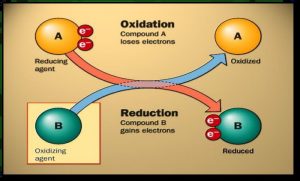
Oxidation and reduction by losing and gaining the electrons
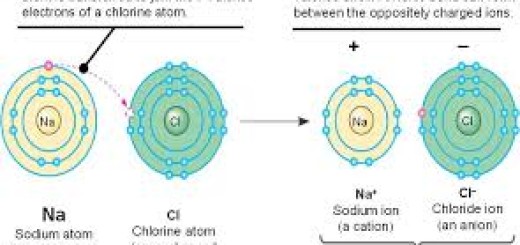
The reaction of sodium with chlorine to produce sodium chloride (the table salt) shows the oxidation and the reduction reaction (It does not include oxygen or hydrogen), Sodium metal and chlorine gas react sharply to form sodium chloride, The oxidation and the reduction happen together in this reaction.
2 Na + Cl2 → 2 NaCl
Sodium atom is monovalent because it loses one electron and changes into the positive (+ ve) ion, This process is known as the oxidation process, Sodium atom is oxidized because it loses an electron and changes into (+ ve) ion, Sodium atom is considered as a reducing agent because it loses (gives) an electron.
Na → Na + + e −
2 Na → 2 Na + + 2 e −
Chlorine atom is monovalent because it gains an electron (which is lost from sodium atom) and changes into a negative (- ve) ion, This process is known as the reduction process, Chlorine atom is reduced because it gains an electron and changes into (-ve) ion, Chlorine atom is considered as an oxidizing agent because it gains an electron.
Cl 2 + e − → Cl −
Cl 2 + 2 e − → 2 Cl −
The ions cannot remain free, therefore the negative (Cl −) ion combines with the positive (Na +) ion to form a sodium chloride molecule (NaCl).
Oxidation is a chemical process where the atom loses an electron or more, The reduction is a chemical process where the atom gains an electron or more during a chemical reaction.
The oxidizing agent (factor) is the substance that gains an electron or more during a chemical reaction, The reduction agent (factor) is the substance that loses an electron or more during a chemical reaction.
Oxidation and reduction reactions according to Traditional concept & Electronic concept
Chemical reactions, Types of thermal decomposition reactions & Air bags importance
The movement of electrons around the nucleus and the energy levels
Simple and double substitution reactions, Reaction between an acid & a salt