Properties of metals during chemical reactions & reaction of active metals
The metals
Metals are the elements that have less than four electrons in their outermost energy levels, During the chemical reactions, the atoms of the metallic elements tend to lose their outermost electrons, and change into positive ions.
The positive ions carry a number of positive charges equal to the number of the lost electrons, The electronic structure of the positive ion is similar to that of the nearest preceding inert gas in the modern periodic table.
Some metals such as Magnesium (Mg) and iron (Fe) react with oxygen giving the metals oxides which are called “Basic oxides”, Basic oxides are metallic oxides, some of them dissolve in the water forming alkaline solutions.
Some metal oxides (such as magnesium oxides) dissolve in the water giving alkalis (metal hydroxide) which turn the litmus solution into blue, Some metal oxides such as iron II oxide do not dissolve in the water. All alkalis are bases, while not all the bases are alkalis.
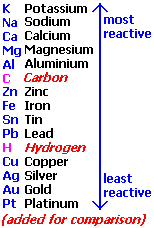
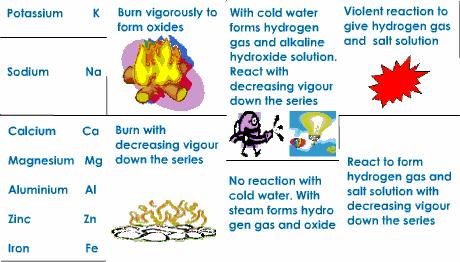
The reaction of the metals with the water depends on the position of the metal in the chemical activity series, The chemical activity series is a series in which metals are arranged in descending order according to their chemical activity.
Potassium and Sodium react instantly with water and hydrogen gas evolves which burns with a pop sound. Calcium and Magnesium react very slowly with cold water. Zinc and Iron react with hot water vapour at high temperatures only. Copper and Silver do not react with water.
Some active metals such as magnesium (Mg) and zinc (Zn) react with dilute acids (such as hydrochloric acid) giving salt of acid and hydrogen gas evolved. The inactive metals such as copper (Cu) do not react with dilute acids and no gas evolves.
You can download Science online application on google play from this link: Science online Apps on Google play
Metallic & nonmetallic property, Acidic & basic property in the periodic table
Modern periodic table and classification of Elements
Radius property, Ionization potential, Electron affinity & Electronegativity
Chemical combination, Properties of Metals, Nonmetals & Noble (inert) gases
Elements of s-block, Properties of the first group elements 1A (Alkali metals) in the periodic table