Simple and double substitution reactions, Reaction between an acid & a salt
The substitution reactions are the reactions that depend on the activity of metals, where the element which is more active substitutes (replaces) the less active one in another compound, The activity of metals can be determined by using the chemical activity series.
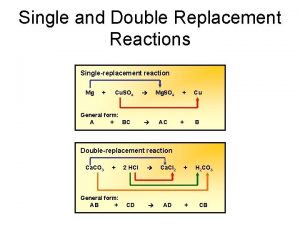
Substitution reactions
The chemical activity series is the arrangement of metals in descending order according to the degree of their chemical activity, All the elements above hydrogen in the series replace hydrogen in acid solutions, whereas the elements that follow hydrogen do not replace hydrogen in acids except under certain conditions, The substitution reactions are classified into two types which are the simple substitution reactions, and the double substitution reactions.
Simple substitution reactions
The simple substitution reactions are the chemical reactions in which one of the elements substitutes other elements in a solution of one of its compounds when the substituting element is more active compared to the substituted one.
A metal substitutes the hydrogen of water and acid
The active metals substitute hydrogen of water and produce metal hydroxide and hydrogen gas evolves, The active metals substitute hydrogen of an acid and produce the salt and hydrogen gas evolves.
The substitution of metal to hydrogen of water
When you put a small piece of sodium by using tongs in a glass of water, Sodium burns with a pop sound (explosion), Sodium reacts with the water (strongly) giving sodium hydroxide and hydrogen gas evolves which burns with a pop sound.
2 Na + 2 H2O → 2 NaOH+ H2 ↑+ heat
The substitution of metal to hydrogen of acid
When you put a small amount of zinc in a beaker, few aluminium sheets in the second beaker and a few copper turnings in the third beaker, then pour a little amount of dilute hydrochloric acid to each beaker.
You notice that the reaction takes place in the zinc beaker immediately, while in the aluminium beaker, It happens after a short time, No reaction takes place in the copper beaker, Zinc and aluminium replace the hydrogen of acid, Copper does not replace the hydrogen of acid.
Although aluminium comes before zinc in C.A.S., It takes more time than zinc to react with the acid due to the presence of a layer of aluminium oxide on aluminium sheets which takes time to separate from aluminium then aluminium becomes exposed to the acid.
Zn + 2 HCL → ZnCl2 + H2 ↑
2 Al + 6 HCL → 2 AlCl3 + 3 H2 ↑
Cu + HCL → No reaction
The metals that come before hydrogen in the chemical activity series can substitute hydrogen of dilute acids forming acid salt and hydrogen gas, The substitution becomes easier as the metal comes much earlier than hydrogen in the series, The metals that come after hydrogen in the chemical activity series can’t substitute hydrogen of dilute acids (except under certain conditions).
A metal substitutes another one in its salt solution
Some metals replace other metals (in their solutions) that follow them in the chemical activity series, when you put some pieces of magnesium ribbon in a beaker containing blue copper sulphate solution, The blue colour of copper sulphate disappears and a reddish-brown precipitate is formed.
Magnesium is more active than copper (as it comes before in the chemical activity series) so, It replaces copper in the copper sulphate solution to form magnesium sulphate ( colourless ) and copper precipitates ( reddish brown ppt.).
Mg + CuSO4 → MgSO4 + Cu ↓
When you put some pieces of copper in sodium sulphate solution, No reaction takes place, because copper is less active than sodium as it comes after sodium in the chemical activity series.
You know that both magnesium and zinc can replace copper in copper sulphate solution as both magnesium and zinc are more active than copper as they come before Cu in C.A.S., Copper can’t replace zinc in its salt solution because copper is less active than zinc as it comes after Zn in C.A.S.
Double substitution reactions
Double substitution reactions are the reactions in which double substitution (exchange) occurs between the ions (radicals) of two compounds to give two other new compounds.
The reaction between an acid and an alkali (Neutralization reaction)
The neutralization reaction is the reaction between an acid and an alkali to form the salt and the water, such as the reaction between hydrochloric acid and sodium hydroxide to produce sodium chloride (salt) and the water.
HCl + NaOH → NaCl + H2O
On heating the products (resultants) of the previous reaction, H2O evaporates and HCl (table salt) remains.
The reaction between an acid and a salt
Acids react with salts and the resultant depends on the type of both the acid and the salt, Hydrochloric acid reacts with sodium carbonate forming sodium chloride, water, and carbon dioxide which turbid the limewater.
Na2CO3 + 2 HCL → 2 NaCl + H2O + CO2 ↑
The reaction between two solutions
The reaction between two salt solutions is accompanied by the formation of precipitates such as the reaction between sodium chloride solution and silver nitrate solution, sodium chloride solution reacts with silver nitrate solution to give sodium nitrate solution and a white precipitate of silver chloride.
NaCl + AgNO3 → NaNO3 + AgCl ↓
Substitution reactions, Chemical activity series, types of simple & double substitution reactions
Types of chemical reactions and Thermal decomposition reactions
Balanced chemical equations, Law of conservation of matter (mass) & Law of constant ratios