Alkenes or Alkylene (Olefins) uses, physical and chemicals properties
Unsaturated open chain aliphatic hydrocarbons are classified into two groups, the Alkenes group which is characterized by the presence of a double bond or more in the carbon chain, Alkynes group which is characterized by the presence of a triple bond in the carbon chain.
Alkene or Alkylene (Olefins)
Alkenes (Olefins) are open-chain aliphatic hydrocarbons, their general formula is (CnH2n), where n is the number of carbon atoms, Total number of atoms = 3n, Olefins are hydrocarbons in which one double bond or more are found between the carbon atoms of their molecules, Alkenes are considered as Alkanes derivatives by removing two hydrogen atoms from the corresponding alkane, therefore, they form a homogeneous series.
The name of alkene is derived from the corresponding alkane by replacement of the ending ane by ene or ylene, Alkenes are characterized by the presence of a double bond between carbon atoms, one of them is a strong sigma bond (σ) and the other is weak pi bond (π) which is easy to be broken, this explains the activity of alkenes.
Alkanes are saturated compounds whereas alkenes are unsaturated compounds because the bonds of alkanes are sigma bonds while alkenes contain pi bonds, Alkenes and alkynes don’t dissolve in water because they are non-polar compounds, and are insoluble in polar solvents, Alkenes are more active than alkanes because they contain unsaturated double bond or more which are broken easily.
Alkenes have a wide range of applications in industry, including the production of polymers, such as polyethylene (PE) and polypropylene (PP), which are used in a variety of products from plastic bags to clothing. As starting materials for the synthesis of other organic compounds, such as alcohols, aldehydes, and ketones. As fuels, such as ethylene, which is a component of natural gas.
Nomenclature of alkenes
Choose the longest continuous carbon chain, the suffix (ane) is replaced by the suffix (ene) in the name of the alkene, preceded by the number of the carbon atom nearest to the double bond, Numbering the carbon chain starts from the nearest side to the double bond regardless the position of the other group.
Ethene (Ethylene) C2H4
Preparation of Alkene by catalytic dehydration of Alcohol: Ethene can be prepared by removing water from ethyl alcohol by using hot concentrated (H2SO4) at 180°C, The function of sodium hydroxide in the apparatus is to neutralize the vapours of H2SO4.
C2H5OH (l) → C2H4 (g) +H2O (l)
This reaction takes place in two steps:
Ethanol reacts with concentrated heated sulphuric acid to form ethyl hydrogen sulphate.
C2H5OH + H2SO4 → C2H5OSO3H+ H2O
Ethyl hydrogen sulphate thermally decomposed to give ethene.
C2H5OSO3H → C2H4+ H2SO4
Economic importance of ethene: It is used in the preparation of polyethene and ethylene glycol which is used in the manufacture of ( dacron fibers, photographic films, cassette tapes).
Physical properties of alkenes
The first members of Alkenes are gases from C2 to C4, alkenes containing 5: 15 carbon atoms are liquids, and Alkenes containing over 15 are solids, Alkenes are non-polar compounds, insoluble in water but they can dissolve in organic solvents such as ether, benzene and carbon tetrachloride.
Chemical properties of alkenes
Alkenes are more active than alkanes due to the presence of the weak pi bond (π), which are easy to be broken.
Burning
Alkenes burn in the air through exothermic chemical reaction giving carbon dioxide and water vapour.
C2H4 + 3O2 → 2CO2 + 2H2O + Energy
Addition reaction
It is the addition of a molecule to an unsaturated compound, where the pi-pond is broken down and saturated compounds are formed.
Addition of Hydrogen “Hydrogenation”
Hydrogenation is the addition of hydrogen to unsaturated hydrocarbons in the presence of a catalyst (Ni or Pt) and the corresponding alkane is formed, Alkenes react with hydrogen in the presence of a catalyst such as nickel or platinum with heating (150°C – 300°C) and the corresponding alkane is formed, in each mole of hydrogen, each pi (π) bond need mole of hydrogen to break it.
C2H4 + H2 → C2H6
Hydrogenated oils (artificial fats): The vegetable oils as maize oil, sunflower oil contain unsaturated compounds, therefore, their melting points are low and they exist in liquid state at normal temperature even they are kept in refrigerator, The hydrogenation of these oils in the presence of Ni make them solidify due to the conversion of unsaturated compounds to saturated compounds.
Addition of Halogen “Halogenation”
When bromine solution, which is dissolved in carbon tetrachloride (CCl4) is added to alkene, the red colour of bromine is removed, since it reacts by addition and pi bond (π) is broken, The reaction is used to detect the presence of double bond where the red colour of bromine is removed.
Halogens react with alkenes by addition, this reaction is used to detect unsaturated alkenes, when bromine in carbon tetrachloride (CCl4) is added, the red colour of bromine is discharged when it is added to ethylene due to the formation of a colourless 1,2 dibromo ethane which is colourless.
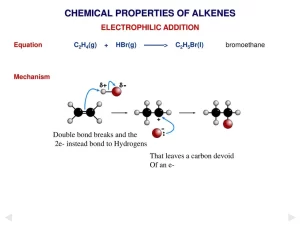
Addition of hydrogen halide (HX) to Alkene
Alkenes react by addition with hydrogen halides where (pi) bond breaks down, the hydrogen atom is added to one carbon atom of the double bond and the halogen atom is added to the other one, giving the corresponding alkyl halide, the addition products depend on the type of alkene.
If the alkene is symmetrical (the two carbon atoms that are attached to the double bond carry the same number of hydrogen atoms), the hydrogen atom is added to any carbon atom and the halogen atom is added to another one.
If the alkene is asymmetrical (the two carbon atoms which attached to the double bond carries different number of hydrogen atoms) the hydrogen atom is added to the carbon atom rich in hydrogen atoms, whereas the halogen atom is added to the other carbon poor in hydrogen, this rule is known as “Markownikoff’s Rule”.
Markownikoff’s Rule is the addition of an asymmetric reagent [H+X− or H2SO4 (H+OSO3H‾)] to an asymmetric alkene as (propane), the positive part of the reagent is added to the carbon atom which carries a large number of hydrogen atoms and the negative part is added to the carbon atom which carries less number of hydrogen atoms.
On adding hydrogen bromide (HBr) to propane, the formula 1-bromo propane CH3CH2CH2Br is not formed, because according to Markownikoff’s Rule, If the alkene is asymmetrical, the hydrogen atom is added to the carbon atom which is rich in hydrogen atoms whereas the hydrogen atom is added to the other carbon which is poor in hydrogen atoms.
Addition of water (H2O) (Catalytic hydration)
Since water is a weak electrolyte, the concentration of Hydrogen ions (H+) is very weak and it is unable to break down the double bond, therefore, the reaction takes place in the presence of strong acid to increase the concentration of hydrogen ions, sulphuric acid is added firstly to ethene to form ethyl hydrogen sulphate which is hydrolyzed to give ethyl alcohol.
C2H4 + H2O → C2H5 OH
The addition of water to ethene takes place in an acidic medium to increase the concentration of H+ which can break down the weak pi-bond.
Oxidation
Alkenes are oxidized by an oxidizing agent such as hydrogen peroxide (H2O2) or alkaline potassium permanganate (KMO4) forming dihydroxy aliphatic compounds known as glycols.
Baeyer’s reaction is the reaction between ethene and potassium permanganate in an alkaline medium where the purple colour of potassium permanganate is discharged, this reaction is very important to detect the double bond.
Oxidation of ethylene with alkaline potassium permanganate (KMO4) where the pi bond (π) between the unsaturated carbon atoms is broken and the two hydroxyl groups are added forming dihydroxy compounds known as glycols (ethylene glycol).
C2H4 + H2O + [O] → C2H6O2
This reaction is used to detect the presence of double bond (unsaturated) where the violet colour of permanganate is removed as it is reduced proving the presence of pi bond (π).
Ethylene glycol is used as antifreeze substance which prevents the freezing of water in car radiators where it forms hydrogen bonds with the water molecule and prevents their combination with each other in the form of ice crystals.
Polymerization
Polymerization is the combination of a huge number of unsaturated simple molecules (monomers), their number ranges from 100 to 1000,000 to form a large molecule (polymer) which has the same empirical formula of the original compound, Empirical formula is the formula which shows the smallest ratio of atoms inside the molecule.
Polymerization takes place for small unsaturated molecules, Each small unsaturated is called Monomer, When two monomers combine together, they form Dimer, When a dimer combine with a monomer, they form Trimer, When a huge number of monomers combine together, they form Polymer, Alkanes don’t form polymer because they are saturated hydrocarbons.
There are two principal methods for the polymerization process which are Addition polymerization and Condensation polymerization, Alkenes are characterized by their ability to form polymers by addition.
Addition polymerization takes by the combination of a similar huge number of unsaturated small molecules (monomers) to each other to form a very large molecule (polymer) such as the formation of polyethylene.
Example: When ethene is heated under high pressure (1000 atmosphere) in the presence of oxygen or hydrogen peroxide H2O2 as initiator substance, polyethylene is formed, its molecular formula reaches to about 30,000 (notice that the molecular mass of ethene 28 only).
In the addition polymerization of ethene, Pi bond is broken and the electrons of this bond are liberated, Each carbon atom now has a free electron, then the carbon atoms of each molecule combine by their free electrons with those of other molecules by a single covalent bond to form a long chain of polymer molecules.
Condensation polymerization takes place between two different monomers and it is accompanied by losing a simple molecule such as water, the copolymer formed is considered as the basic unit that continues the polymerization process.
Uses of some polymers
- Polyethylene: Plastic – Bags – Bottles – Hoses.
- Polypropylene (pp): Carpets – Cases – Cans.
- Polyvinyl chloride (PVC): Drainage tubes – plastic tubes – shoes – Hoses – Electrical wires insulators – Floors – oil bottles.
- Poly tetra fluoro ethylene (Taflon): lining cooking utensils – surgical threads.
Alkanes economic importance, use, physical and chemical properties
Alkynes (Acetylenes) nomenclature, uses and chemical properties