The coordinate bond, Physical bonds (hydrogen bond and the metallic bond)
The coordinate bond is considered a special type of covalent bonds that differs only in the origin of the electron pair which is shared between the atoms involved in the covalent bond, because the electron pair forming the ordinary covalent bond is produced by both of the two combined atoms contributing one electron to the bond, whereas the electron pair of the coordinate bond is produced only by one of the two combined atoms.
The coordinate bond
The coordinate bond is a bond that is formed between a donor atom having a lone pair of electrons or more and an acceptor atom having a vacant orbital, The coordinate bond is represented by an arrow directed from the donor atom to the acceptor atom, The coordinate bond is formed between two atoms:
- The first: having a lone pair of electrons occupying one orbital, This atom is called the donor atom.
- The second: having a vacant orbital, needing this electron pair to acquire the stable electron structure, This atom is called the acceptor atom.
Application 1: The coordinate bond formed in the ammonium ion (NH4)+
Ammonia gas dissolves in water forming a coordinate bond between:
- Nitrogen atom of ammonia molecule which contains a lone pair of electrons, so, the nitrogen atom represents the donor atom.
- Water proton (positive hydrogen ion H+) which contains a vacant orbital accepts the lone pair of electrons from nitrogen atom, so, the proton H+ represents the acceptor atom.
Application 2: The coordinate bond formed in hydronium ion (H3O)+
Acids dissolve in water forming a coordinate bond between:
- Oxygen atom of the water molecule which contains a lone pair of electrons, The oxygen atom represents the donor atom.
- Acid proton (positive hydrogen ion H+) which contains a vacant orbital accepts the lone electron pair from oxygen atom, The proton H+ represents the acceptor atom, It is observed that the other lone electron pair remains as it is in the oxygen atom.
There are no positive hydrogen ions (protons) in aqueous solutions of the strong acids, Because of their bonding with water molecules by coordinate bonds forming hydronium ions (H3O+).
Ammonium hydroxide molecule (NH4OH) contains three types of the bonds because it contains:
- Ionic bond as a result of an electrostatic attraction occurs between the positive ammonium ion (NH4)+ and the negative hydroxide ion (OH−) .
- Coordinate bond in ammonium ion, when the nitrogen atom of the ammonia molecule donates the lone electron pair to the positive proton (H+).
- Three covalent bonds in ammonia molecule resulting from the sharing of electrons between the nitrogen atom and the three hydrogen atoms.
Physical bonds
Physical bonds are the hydrogen bond and the metallic bond.
The hydrogen bond
It is known that water boils at 100° C, This temperature is considered to be high for a compound of a relatively low molecular mass (18 g/mol).
If this temperature is compared with the boiling point of hydrogen sulphide (of molecular mass 34 g/mol) which is – 61° C, we will find a great difference although oxygen precedes sulphur in the same group 6A in the periodic table.
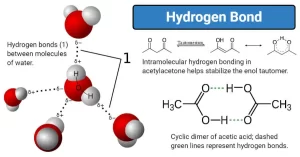
In general, as relative molecular mass increases, the boiling point increases, but the boiling point of water is anomalous in the group (VI) hydrides, This anomalousness of the boiling point of water is explained by the attraction of water molecules to each other by what is known as the hydrogen bond or hydrogen bridge.
To form a hydrogen bond, the hydrogen atom must become two atoms of high electronegativity such as (N, O, F atoms), The hydrogen bond is the bond that is formed between hydrogen atom binds by a polar bond [eg. : (F − H), (O − H), (N − H)] with high electronegative bonded atom [eg. : F, O, N], All the compounds of hydrogen bonds are polar compounds dissolve in polar solvents such as water.
Examples:
- The bond between hydrogen fluoride molecule HF.
- The bond between water molecules H2O.
- The bond between ammonia molecules NH3.
Application: Hydrogen bonds between water molecules
- The oxygen atom in water carries a partial negative charge (δ− −), The two hydrogen atoms carries a partial positive charge (δ++).
- The two hydrogen atoms become as a bridge between the two oxygen atoms which have a high electronegativity.
- The molecules get near to each other through the hydrogen atom which bonded the water molecules together.
The high boiling point of water, due to the consumption of a big amount of heat energy to break the hydrogen bonds between water molecules.
The strength of hydrogen bond
Although the hydrogen bond clearly affects physical properties of the compounds (as water), its bond strength is far weaker than the covalent bond, because the length of the hydrogen bond is longer than that of the covalent bond, as the length of the bond increases and the bond strength becomes weaker.
The strength of hydrogen bond increases, when the hydrogen bond becomes in a straight line with the polar covalent bond as in water molecules H2O, Hydrogen fluorine molecules HF, By increasing the difference in the electronegativity between a hydrogen atom and the other atom in the polar covalent bond.
The hydrogen bonds between hydrogen fluoride molecules (HF) are stronger than that between water molecules, Because the difference in the electronegativity between fluorine & hydrogen is greater than that between oxygen & hydrogen, the strength of the hydrogen bond increases as the difference in the electronegativity between the bonded atoms increases.
The hydrogen bond between H2O molecules is stronger than that between NH3 molecules, Because the difference in the electronegativity between nitrogen & hydrogen atoms, In addition, the hydrogen bonds in water molecules are in a straight line with the polar covalent bond.
The forms of the compounds which have hydrogen bonds
The compounds which have hydrogen bonds take several forms, the compounds may form Straight line such as the hydrogen bonds between hydrogen fluorine molecules, closed ring such as the hydrogen bonds between hydrogen fluorine molecules, Open net such as the hydrogen bonds between water molecules.
The metallic bond
Each metal has a crystal lattice with a definite form in which the positive metal ions take a certain arrangement, The outermost shell electrons (valence electrons) of each atom are associated together forming an electron cloud with free movement to bind this great collection of positive metal ions by what is known as a metallic bond.
The metallic bond is the bond produced from the electron cloud of valence electrons which decreases the repulsive forces between the positive metal ions in the crystal lattice, These free valence electrons account for the good electrical and thermal conductivity of metals.
The metallic bond strength
The number of valence electrons in the metal atom plays an important role in the strength of the metallic bond, as the number of valence electrons increases in the metal atoms, the atoms become more strongly bound and accordingly the metal becomes harder and has a higher melting point.
Hardness: The strength of metal to resist scratch, the scientist Friedrich Mohs had put a scale for it, The hardness of tale (made of magnesium silicate) = 1 and can be scratched by fingernails, The hardness of diamond = 10 and can be scratched by another metal.
Molecular orbital theory & Valence shell electron pair repulsion (VSEPR) theory
Elements of s-block, Properties of the first group elements 1A (Alkali metals) in the periodic table