Phenols importance, preparation, physical and chemical properties
Phenols are hydroxy aromatic compounds where one or more hydroxyl groups are directly attached to the carbon atoms of the benzene ring, Phenol is an organic compound that has great industrial importance, It is used as a starting material for the synthesis of many aromatic compounds such as polymers, dyes, disinfectants, salicylic acid derivatives (as aspirin) & picric acid.
Phenols
Picric acid is an explosive substance and it is used in the treatment of burns, Phenols (Carbolic acid) can be considered as aryl derivatives of water, and Phenol can be considered as derivatives of aromatic hydrocarbons by substitution of a hydrogen atom by the hydroxyl group.
Preparation of phenol
- From fractional distillation of coal tar.
- From halogenated aromatic compounds, by the hydrolysis of chlorobenzene with sodium hydroxide at high temperature and high pressure of 300 atmospheres.
C6H5Cl + NaOH → C6H6O + NaCl
Coal → Coal tar → phenol (182°C)
In the preparation of phenol from chlorobenzene, pressure is applied because it is difficult to break the bond between the halogen atom and carbon of the benzene ring.
Physical properties of phenol
Phenol is a solid corrosive substance, It has a characteristic odour, its melting point is 43°C and its boiling point is 182°C, It is sparingly soluble in water and its solubility increases by increasing the temperature (completely soluble with water at 65° C), It is colourless and crystalline substance with an odour characteristic.
Chemical properties of phenol
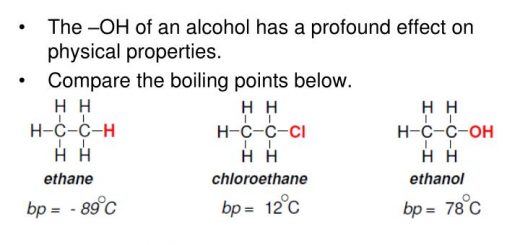
The acidic properties are attributed to the presence of hydrogen ion, Both alcohols and phenol react with strong metals such as sodium and hydrogen gas is evolved, this is due to the polarity of (O−H) bond.
The polarity of (O−H) bond increases in phenols as the benzene ring in phenol increases the polarity which makes the bond (O−H) longer and weaker which facilitates the separation of hydrogen ion, that is why phenol reacts with the alkalis like caustic soda and this doesn’t take place in alcohols, and it is considered as an acid named in industry carbolic acid.
Benzene ring affects the bond between the carbon atoms of the benzene ring in phenol and the oxygen atom of the hydroxyl group, this makes the bond shorter and stronger so, it is impossible to eliminate the hydroxyl group from phenol when it reacts with acids while alcohols react easily with acids.
Ethanol reacts with hydrochloric acid while phenol doesn’t react because hydroxyl group is removed easily from alcohols while it is difficult to remove it from phenols, The acidity of phenol is more than that of ethanol because the benzene ring increases the length of (O−H) bond and weakens it, so the hydrogen ion is separated easily while the alkyl group of alcohols decreases the length of (O−H) bond and strengthens it so, it is difficult to remove H+.
The reaction of phenol with acetic acid:
CH3COOH + HOC6H5 → CH3COOC6H5 + H2O
Reaction specific to the hydroxyl group: Phenols do not react with HX in normal conditions due to the strong bond between carbon of the benzene ring and the hydroxyl group, so it is impossible to eliminate the hydroxyl group from phenol.
Reaction specific to the carbinol group: Phenol does not oxidize chemically.
Reaction specific to benzene ring
Nitration: Phenols reacts with concentrated nitric acid in the presence of conc. H2SO4 to form 2,4,6-tri-nitro phenol which is known commercially as picric acid, Picric acid is used in the manufacture of explosives, treatment of burns, It gives the skin a yellow colour, which is difficult to be removed and it remains for several days until the skin (outer layer) renewed.
C6H6O + 3HNO3 → C6H3O7N3 + 3H2O
With formaldehyde: Phenol reacts with formaldehyde in acidic or alkaline medium to form a copolymer, then the polymerization process takes place condensation to form bakelite polymer.
Polymerization by condensation
These copolymers are produced usually from the combination of two types of monomers, with the elimination of a small molecule such as water molecule, The 1st step begins by the reaction between the molecule of formaldehyde with two molecules of phenol where H2O molecule is produced, then the molecules of copolymer combine together in sequence to form a network polymer called Bakelite in which a copolymer with three dimensions is formed, Each two phenol molecules are linked by (− CH2) bridge.
Phenol + HCHO + Acidic or Alkaline medium → Bakelite
Bakelite is a type of thermosetting crosslinking plastic which resists heat, Bakelite has a brown colour, It is used as a good insulator of electricity, So, it is used in the manufacture of some electric instruments and ashtrays, Bakelite is the copolymer produced from the condensation of phenol with formaldehyde.
Detection of phenol
- By adding a few drops of iron III chloride solution to phenol, violet colour is produced.
- By adding bromine water to phenol, a white precipitate is produced.
The acidity of alcohols and phenols
Alcohols have a weak acidic character, especially with strong active metals, this is related to the presence of the polar covalent bond which combines the hydrogen atom to oxygen atom in the hydroxyl group, The electron pair is shifted towards the oxygen atom, this facilitates the break down of the polar covalent bond, so the metal atom can replace the hydrogen of the hydroxyl group.
Phenols have an acidic character more than that of alcohols due to the polarity of (O−H) bond which increases in phenols due to the presence of benzene ring which leads to the weakness of (O−H) bond which facilitates the separation of hydrogen ion.
Chemicals properties of alcohols, Economic importance of Ethanol, Ethylene Glycol & Glycerol
Acetic acid, Aliphatic carboxylic acids types, nomenclature, preparation & general properties