Acid-base disturbances, metabolic acidosis causes, Effects of acidosis and alkalosis on the body
The bicarbonate buffer system is the most important buffer in the body because the concentrations of its components can be independently regulated, PCO2 by the lungs and PHCO3− by the kidneys. The lungs and kidneys can also cause an abnormal pH by changing PCO2 and PHCO3− respectively.
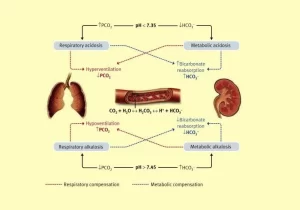
Respiratory acidosis
The primary abnormality is an increase in arterial PCO2; a major cause of respiratory acidosis is hypoventilation.
Causes of hypoventilation
- Damage to the respiratory center in the medulla oblongata.
- Obstruction of air passages.
- Pneumonia.
- Decreased pulmonary membrane surface.
Respiratory acidosis is compensated by the kidneys which increase the secretion of H+ and increase the generation of HCO3, i.e. (HCO3−) P increase, Thus, although PCO2 remains elevated the P (HCO3−) increases and so the pH increases towards normal.
Respiratory alkalosis
The primary abnormality is a decrease in arterial P (CO₂). It results from hyperventilation.
Causes of hyperventilation
- Psychoneurosis (causes overbreathing)
- High altitude
Respiratory alkalosis is compensated by the kidneys, The kidneys not only fail to generate any new HCO3− but also fail to reabsorb all the filtered HCO3, (HCO3) P falls, and although the arterial PCO2 remains low, the ratio decreases towards normal.
Metabolic acidosis
The primary abnormality is a decrease in P (HCO3−), Most cases of metabolic acidosis result from the abnormal accumulation of organic acids.
Causes of metabolic acidosis
- Diarrhea: the gastrointestinal secretions normally contain large amounts of sodium bicarbonate, Therefore, excessive loss of these secretions during a bout of diarrhea is exactly the same as the excretion of a large amount of sodium bicarbonate into the urine.
- Hypovolemia and other forms of circulatory shock in which lactic acid may accumulate (lactic acidosis).
- Uncontrolled diabetes mellitus: in which the acetoacetic acid and B-OH-butyric acid may accumulate (Diabetic ketoacidosis).
- Severe renal failure (uraemia): failure of the kidneys to rid the body of even the normal amounts of acid formed each day by the metabolic processes of the body.
The decreased arterial pH stimulates chemoreceptors in the carotid bodies, which in turn initiate are flex stimulation of alveolar ventilation, The increase in alveolar ventilation results in a decrease in arterial (CO₂)P, Thus, although (HCO3−) P remains low, the (HCO3−)P/PCO₂ and hence arterial pH increases 50%- 70% towards normal, Metabolic acidosis is compensated by the lungs, then the kidneys act to correct the PH back to normal.
Metabolic alkalosis
The primary abnormality is an increase in (HCO3-)P.
Causes of metabolic alkalosis
- Excessive ingestion of alkaline drugs: such as sodium bicarbonate for the treatment of gastritis or peptic ulcer.
- Excessive vomiting of gastric contents.
- Hyperaldosteronism: Aldosterone promotes excessive reabsorption of sodium ions from the distal segments of the tubular system, coupled with increased secretion of hydrogen ions, thus promoting alkalosis.
The increased arterial pH is sensed by the chemoreceptors in the carotid bodies, which in turn initiates a reflex decrease in alveolar ventilation, Thus, there will be an increase in arterial PCO2, and the (HCO3−)P/PCO₂ ratio will decrease 50% -70% to normal, Metabolic acidosis is compensated by the lungs, and then the kidneys react and correct the pH back to normal.
Effects of acidosis and alkalosis on the body
Acidosis
The major effect of acidosis is the depression of the central nervous system, Therefore, patients dying of diabetes, uremic, and other types of acidosis usually die in a state of coma, In metabolic acidosis, the high hydrogen ion concentration causes an increased rate and depth of respiration, Therefore, one of the diagnostic signs of metabolic acidosis is increased pulmonary ventilation, On the other hand, in the respiratory acidosis, the respiration is usually depressed because this is the cause of acidosis.
Alkalosis
The major effect of alkalosis on the body is the over-excitability of the nervous system, This occurs in both the central nervous system and in the peripheral nerves, but usually, the peripheral nerves are affected before the central nervous system, The nerves often become so excitable that they automatically and repetitively fire even when they are not stimulated by normal stimuli, As a result, the muscles go into a state of tetany.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science Online application on Google Play from this link: Science Online Apps on Google Play
Urinary system development, function & Congenital Malformations of the kidney
Acid-base balance importance, Defense against changes in Hydrogen ion & Threats of pH
Physical properties of urine, Tests to evaluate kidney function & Normal constituents of urine
Urine formation, Factors affecting Glomerular filtration rate, Tubular reabsorption & secretion
Histological structure of kidneys, Uriniferous tubules & Types of nephrons
Functions of Kidneys, Role of Kidney in glucose homeostasis, Lipid & protein metabolism