Chemical structure and importance of living organisms’ bodies (carbohydrates)
Biological macromolecules (The polymers)
They are large organic compounds made up of smaller molecules (monomers) combined together by polymerization process, polymerization is a process by which monomers are combined together to form the polymer, The polymers are biological macromolecules formed by the combination of smaller molecules throughout polymerization process such as proteins, lipids and carbohydrates .
The structural sequence of the living organisms
The living organisms’ bodies consist of the systems, the systems are formed of organs that are formed of tissues, The tissues are formed of the cells, The cells are formed of organelles that are formed of molecules , Molecules are formed of atoms.
Classifications of molecules that enter in the living organisms’ cells
All cells of living organisms are made up of Organic compounds and Inorganic compounds, Organic compounds are compounds that mainly contain carbon (C) and hydrogen (H) atoms, They may contain other elements such as oxygen and nitrogen such as Biological macromolecules that include the carbohydrates, lipids, proteins and nucleic acids, Inorganic compounds are compounds that do not contain carbon atoms, Such as water H2O and Mineral salts ( such as NaCl ).
Classification of the biological macromolecules
The biological macromolecules are classified into four groups according to their molecular structure and the functions they perform , These four groups are Carbohydrates , lipids, proteins and nucleic acids .
Carbohydrates
They are biological macromolecules ( polymers ) made up of many smaller molecules ( monomers ) called the monosaccharides , They include the sugars , starches and fibres .
Carbohydrates are made up of carbon (C), hydrogen (H) and oxygen (O) atoms in a ratio (1: 2: 1), General formula of Carbohydrates: (CH2O)n , such as Glucose C6H12O6.
Carbohydrates Classification
The carbohydrates are classified according their molecular structure into simple sugars (Monosaccharides, Disaccharides) and the complex sugars (the polysaccharides).
Monosaccharides such as Glucose , Fructose , Galactose & Ribose, Disaccharides such as lactose , Maltose & Sucrose, Polysaccharides such as Starch , Cellulose & Glycogen .
Simple sugars
Properties : They are water soluble , They have low molecular weight and they have a sweet taste .
Simple sugars types
Monosaccharides
Molecular structure are made up only of one molecule consisted of a chain of carbon atoms ( 3 : 6 atoms ) , each of them is connected to oxygen and hydrogen atoms in a certain way , Therefore , monosaccharides are the simplest type of sugars .
Monosaccharides such as Glucose ( grape sugar ) , Fructose ( fruit sugar ) , Galactose ( made in the glands that produce milk ) , Ribose ( pentose sugar ) ( it has 5 C atoms ) .
Disaccharides
Molecular structure are made up of two molecules of monosaccharides linked together to form a disaccharide molecule .
Disaccharides such as Lactose ( milk sugar ) ( It is formed of Glucose molecule + Galactose molecule ).
Maltose ( malt sugar ) ( It is formed of Glucose molecule + Glucose molecule ).
Sucrose ( cane sugar ) ( It is formed of Glucose molecule + Fructose molecule ) .
Role of monosaccharides in energy transferring processes inside the cells :
During glucose oxidation inside mitochondria :
- The energy that stored in the chemical bonds of glucose is released to be stored in the compound that is called adenosine triphosphate ( ATP ) .
- Adenosine triphosphate ( ATP ) is then transferred to the other places in the cell to use the stored energy in it for performing all vital processes inside the cell.
Complex sugars ( polysaccharides )
They are insoluble in water , They have molecular weight and they do not have a sweet taste, Molecular structure made up of many molecules of monosaccharides linked together, Complex sugars such as Starch , Cellulose and Glycogen ( Each molecule of them consists of glucose molecules linked together by different ways ) .
Life application
Blue Benedict’s reagent is used for detecting simple sugars in the blood and urine.
Diabetic and obese patients must keep themselves away from taking sugary and starchy substances .
Importance of carbohydrates
Carbohydrates are one of basic and fast resources for obtaining the energy .
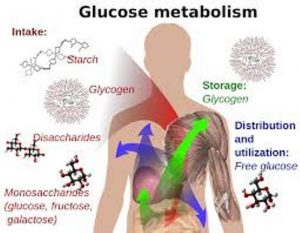
Carbohydrates are used for storing energy in organisms’ bodies until they require it , where plants store carbohydrates in the form of starches , Humans and animals store carbohydrates in the form of glycogen in cells of liver and muscles .
Carbohydrates are the basic component of some parts of the cell, Such as Cellulose enters in the structure of cell walls of plant cells, Carbohydrates enter in the structure of cell membranes and protoplasm .
Carbohydrate Metabolism, Importance & Hormonal regulation of glycolysis
Importance and Chemical structure of living organism’s bodies ( Proteins )
The importance , classification & molecular structure of lipids