Enzymes meaning, function, examples & Mechanism of enzyme action
Enzymes are proteins, they act as biological catalysts (biocatalysts). Catalysts accelerate chemical reactions. they increase the reaction rate by lowering its activation energy, they can make their conversion of substrate to product occur many millions of times faster. they are used in the synthesis of antibiotics, enzymes can be used to speed up chemical reactions: they can break down protein, starch, or fat stains on clothes.
Enzymes
Enzymes are a group of organic catalyst protein in nature, present inside the living cells but they can act independently of the calls. They are of high molecular weight, heat-labile, non-dialyzable, and colloidal in nature.
Some terms used in Enzymology
- Substrate: is the organic substance on which the enzyme acts.
- Product: is the organic substance produced by the action of the enzyme.
- Coenzymes: Essential for the activity of enzymes and they are considered as second substrates or Co-substrate.
Terminology of enzymes
Enzymes that were discovered early were given general names which they are still used as ptyalin, pepsin, trypsin, cathepsin, emulsin, etc… Now a suffix (ase) is added to indicate enzyme. The prefix may be:
- Name indicating general nature of substrate e.g.: protease, lipase etc…
- The real name of the substrate: urease, lactase, sucrase.
- Type of reaction catalyzed e.g.: Aldolase, mutase, transaminase, dehydrogenase etc…
- Combination of the above e.g.: Name of substrate + type of reactions.g.:
lactic acid Dehydrogenase
Glycogen Synthetase
Enzyme specificity
In the contrary to inorganic catalysts which catalyze a wide variety of different reactions, enzymes show different levels of specificity which are:
1- Absolute specificity: In this condition, the enzyme acts only on a specific substrate e.g. Lactase acts on lactose.
2- Group specificity (Structural specificity): In this condition, the enzyme acts on a special type of bond at a specific site and attached to specific groups e.g.:
- Pepsin: acts on peptide bonds between the amino group of aromatic amino acid and the carboxylic group of another amino acid.
- Trypsin: acts on peptide bonds between the carboxylic group of basic amino acid and the amino group of another amino acid.
3- Relative specificity: In this condition, the enzyme acts at different rates on one type of bond in compounds chemically related e.g. pancreatic lipase hydrolyzes the alpha ester bonds at positions 1 & 3 in Triglycerides, (whether these are tristearin, triolein, tripalmitin).
4- Stereochemical specificity: Enzymes act on D or L isomers e.g.
- D-Amino acid oxidase acts only on D-amino acids
- L- Amino acid oxidase acts only on L- amino acids.
Enzymes act on a specific type of linkages according to the type of linkage {α or Β) of the compounds attached to it e.g. Amylase hydrolyses α-1.4 glycosidic linkage of starch.
Clinical importance of enzymes
Diagnosis of diseases
Enzymes are intracellular and when there is cellular damage, they are released into the circulation: the measurement of these enzymes in the serum can be used in the diagnosis of certain diseases.
Enzymes in plasma are classified into:
Functional plasma enzymes: These are enzymes present normally in the blood to perform certain physiological functions. They are characterized by:
- They are synthesized in the liver.
- They are present in blood in higher concentrations than tissues.
- Their substrates are present in the circulation.
Examples: Proenzymes of blood clotting, lipoprotein lipase, and pseudocholine esterase.
Non-functional plasma enzymes: These enzymes present normally in a very low concentration in the blood due to tissue turn over and increases in case of tissue damage. They are characterized by:
- They perform no physiological function in the blood.
- Their substrates are absent from blood.
- Their level is normally low and they increase in tissue damage.
Examples of these enzymes are:
- Alkaline phosphatase: increases in obstructive jaundice, hyperparathyroidism, rickets, and metastatic carcinoma to the bone.
- Transaminases: Aspartate aminotransferase (AST) or glutamic oxaloacetic transaminase (GOT) increases in heart disease. Alanine aminotransferase (ALT) or glutamic pyruvic transaminase (GPT) increase in liver diseases.
- Some enzymes can be used as tumor markers.
Treatment of diseases
Enzymes are used in the treatment of some diseases e.g.
- Fibrinolysins in the treatment of infarctions (streptokinase).
- Digestive enzymes in the treatment of maldigestion.
- α-chymotrypsin for treatment of intraocular haemorrhage.
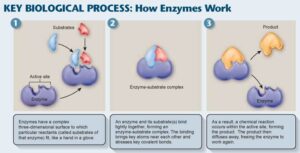
Mechanism of enzyme action
As it was previously said, enzymes are organic catalysts, i.e. they do not initiate the reactions but they enhance them. i.e. enzymes make spontaneous reactions proceed rapidly under the conditions prevailing in living cells. Most of the chemical reactions in the body fail to react rapidly because most of them possess insufficient kinetic energy to overcome the energy barrier for reaction.
Enzymes may be considered to lower energy barriers for chemical reactions i.e. they reduce activation energy. Activation energy is defined as the energy required to convert all molecules in one mole of a reacting substance from the ground state to the transition state.
- Transition state: the unstable arrangement of atoms in which chemical bonds are in the process of being formed or broken.
- Activation energy is the energy required to reach the transition state from the ground state of the reactants.
In order to understand the mode of action of enzymes we have to know some terms:
Catalytic site: is a special sequence of amino acids in the protein molecule of the enzyme to which the substrate is attached.
Active site: This is a more general term that involves all sequences of amino acids that affect the activity of the enzyme i.e it includes, for example, the catalytic site, the allosteric sites, etc….
In general, the mode of action of an enzyme (E) is that: the enzyme combines with the substrate (S) at the catalytic site to form an enzyme-substrate complex (ES), later on, the enzyme is regenerated and the product is formed.
E + S ⇔ (ES) ⇔ E + P
Substrate entering the active site of enzyme ⇒ Enzyme substrate complex ⇒ Enzyme/products complex ⇒ Products leaving the active site of the enzyme
Mode of binding of substrate to Enzyme
Two models are proposed for the formation of the enzyme-substrate complex:
- Rigid model of the catalytic site (Lock and key model): The substrate reacts with the enzymes in terms of a lock and key or rigid template model. The catalytic site can be preshaped to fit the substrate. they are complementary in terms of shape. This type of enzyme is usually highly specific.
- Flexible mode (induced fit model) of the catalytic site: The substrate induces a conformational change in the catalytic site such that it becomes fit to the substrate binding. This makes the amino acids on the catalytic site to take a three-dimensional structure.
Measurement of the rate of enzyme reaction
The rate of enzyme reaction (velocity) is assessed by the rate of change of substrate to product per unit time.
Measurement of enzyme activity, Enzyme unit (U):
Enzyme unit (U): is the amount of enzyme that converts one micromole of substrate per liter of sample per minute. It is abbreviated as U/L.
Isoenzymes structure, Coenzymes function & Classification of enzymes
Factors affecting the rate of enzyme reaction & Importance of enzyme inhibitors
Protein Classification, Globular & Fibrous protein, Simple, Compound & Derived proteins
Protein definition, structure, order, denaturation & Bonds responsible for protein structure
Protein structure, Classification, properties of amino acids & Formation of peptide bonds
DNA Repair types, definition & importance, Protein Biosynthesis & Genetic Code