What are the types of covalent bonds?
The covalent bond
The covalent bond is a bond occurred among the atoms of nonmetals through the participation of each atom with the same number of electrons to complete the outer electron shell.
The covalent bond is a type of bonds that occurs between two nonmetals, when two nonmetals atoms are interacting with each other, no one of each loses or even gains any electrons.
Each atom shares the other with a number of electrons (from its outermost shell) equal to the number of electrons it needs to complete its outermost shell, There is an interference occurred among both atoms, resulting in bond known as the covalent bond.
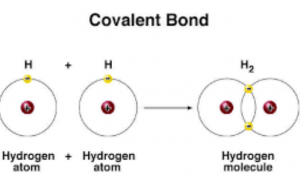
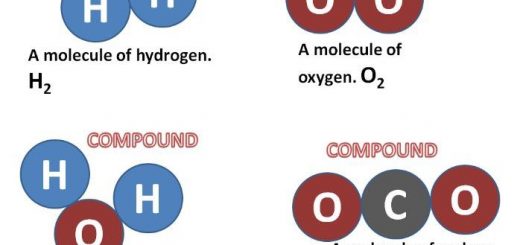
The covalent bond is formed by sharing of one pair of electrons or more, and it may be formed between 2 atoms of the same element, The covalent bond is formed due to sharing of electrons between the atoms, and it produces the element and the compound molecules.
The covalent bonds produce element molecules between two similar nonmetals atoms, they produce compound molecules between two different nonmetallic atoms, The covalent bonds produce the covalent compounds.
The types of the covalent bond
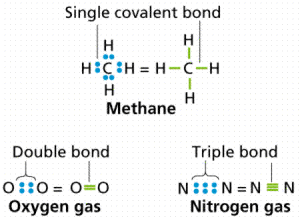
The covalent bond has three types of covalent bond which are single covalent bond, double covalent bond and triple covalent bond.
A single covalent bond is the bond that arises between two nonmetals atoms, where each atom shares the other atom with one electron to complete its outermost shell, and becomes more stable, Single covalent bond is represented by one line ( – ) joining the two atoms.
A double covalent bond is the bond that arises between two nonmetal atoms, where each atom shares the other atom with two electrons to complete its outermost shell with 8 electrons and becomes more stable, Double covalent bond is represented by two lines ( = ) joining the two atoms.
Triple covalent bond is the bond that arises between two nonmetal atoms, where each atom shares the other atom with three electrons to complete its outermost shell with 8 electrons and becomes more stable.
The quantum numbers and principles of distributing electrons
Chemical combination, Types of bonds (Chemical bonds & Physical bonds)
Theories explaining the covalent bond, Octet rule & Overlapped orbitals concept
Chemical bonds, Ionic bonds, Properties & types of covalent bonds