Classifications of bases according to strength (degree of ionization) & molecular structure
Bases can be classifies according to strength ( degree of ionization ) and molecular structure , Bases are classified according to their degree of ionization ( dissociation ) into strong bases and weak bases .
Classifications of bases according to strength ( degree of ionization )
Strong bases
Bases which are completely ionized in the water, Their solutions are good conductors of electricity, They are considered as strong electrolytes .
Examples : Potassium hydroxide KOH , Sodium hydroxide NaOH , Barium hydroxide Ba(OH)2 .
Weak bases
Bases which are incompletely ionized in the water, Their solutions are bad conductors of electricity ,They are considered as weak electrolytes .
Examples : Ammonium hydroxide NH4OH
Classifications of bases according to molecular structure
Bases are classified according to their molecular structure into :
Metal oxides such as Iron ( II ) oxide FeO and Magnesium oxide MgO.
FeO ( s ) + 2HCl ( aq ) → FeCl2 + H2 O ( l )
Metal hydroxides such as Calcium hydroxide Ca(OH)2 , Sodium hydroxide NaOH .
Ca(OH)2 ( aq ) + H2SO4 ( aq ) → CaSO4 ( aq ) + 2 H2 O ( l )
Metal carbonates such as potassium carbonate K2CO3 and Sodium carbonate Na2CO3 .
K2CO3 ( s ) + 2HCl ( aq ) → 2KCl ( aq ) + H2 O ( l ) + CO2 ( g )
Metal bicarbonates such as potassium bicarbonate KHCO3 and Sodium bicarbonate NaHCO3 .
KHCO3 ( s ) + HCl ( aq ) → KCl ( aq ) + H2 O ( l ) + CO2 ( g )
The bases that dissolve in the water are called Alkalis , Alkali is a base that dissolves in the water and gives hydroxide ion ( OH−), So, the alkalis are a part of the bases and therefore, we can say that : all alkalis are bases and not all bases are alkalis .
Sodium carbonate is from bases because sodium carbonate reacts with acid forming salt and water , Not all bases are alkalis because there are some bases don’t dissolve in the water .
Detecting acids and bases
The aqueous solutions are divided into three types which are Acidic solutions , Alkaline solutions and Neutral solutions .
There are two methods for identifying these solutions which are Indicators and pH-meter .
Using the indicators for identifying the aqueous solutions
Indicators are weak organic acids or bases , their colour changes with the change of the solution type.
Indicators are used in identifying the type of solution and determining the end point in titration process between acids and bases.
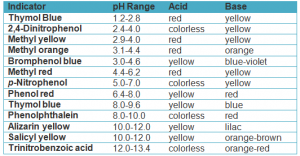
The following table shows examples of some indicators
Ant and bee bites have an acidic effect , and can be treated by using sodium bicarbonate solution , whereas the wasp and jelly fish have a basic effect and can be treated by using vinegar .
The color of indicator changes with the change of the solution type because the color of non-ionized indicator is different from the color of ionized indicator in different solutions .
Phenolphthalein can not be used to differentiate between the acidic and the neutral medium because it has the same color ( colorless ) in both media .
Using the hydrogen exponent pH for identifying the aqueous solutions
The hydrogen exponent pH is a way to express the degree of acidity or alkalinity of a solution , pH value expressed by positive numbers range from 0 to 14 , pH value can be detected by pH paper tape and pH-meter .
The pH value depends on the concentration of positive hydrogen ions ( H+ ) and negative hydroxide ions ( OH− ) in the solutions as follows :
Acidic solution
When the monobasic acid dissolves in the water , each molecule gives one proton H+ , The concentration of H+ > OH− , PH value < 7.
Neutral solution
When NaCl dissolves in the water, The concentration of H+ = OH− , PH value = 7 .
Basic solution
When NaOH dissolves in the water , each molecule gives one hydroxide ion , The concentration of H+ < OH− , PH value > 7 .
Vinegar , lemon and tomato juices are acidic solutions ( pH < 7 ) .
Washing soda , detergents and glair are basic substances ( pH > 7 ) .
Types of compounds, Properties of Acids, Bases (alkalis), Oxides and Salts
Classification of Acids according to its strength (degree of ionization), Its source & Basicity
Economic importance of some common acids, bases and salts (minerals)