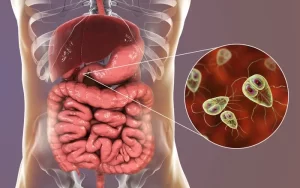
Diagnosis of parasitic infections and Techniques used for identification of Intestinal Parasites
Parasites live off other organisms, or hosts, to survive, Some parasites don’t affect their hosts, and others grow, reproduce, or invade organ systems that make their hosts sick, resulting in parasitic infection. Malaria is one of the deadliest parasitic diseases, Common parasitic infections include trichomoniasis, giardiasis, cryptosporidiosis, and toxoplasmosis.
Diagnosis of parasitic infections
This can be achieved by:
- History taking such as travel history to endemic areas, geographic location of the patients, history of exposure to contaminated water or arthropod bite, family history……etc.
- Clinical diagnosis depends on the characteristic signs and symptoms related to the parasitic infection, e.g. blood in the stool or urine in case of schistosomal infection.
- Laboratory diagnosis can be achieved by the following:
- Direct methods: include macroscopic and microscopic examination of the patients’ samples.
- Indirect methods: include immunological tests.
- Molecular methods.
Laboratory diagnosis of parasitic infections
1. Direct methods for diagnosis of parasitic infections
This is based on the examination of excreta, body fluids, and tissues and the visualization of the different diagnostic stages of parasites. The samples examined are the following:
- Stool.
- Urine.
- Aspirates.
- Sputum.
- Biopsy.
- Blood.
Other additional techniques may aid in diagnosis such as animal inoculation, culture using specific growth media,… etc.
Stool Examination
The collection of samples should be in a wide-mouthed container with a screw top. The sample should not be contaminated with urine or water, since urine can destroy some parasites and water may contain ciliates which can cause false diagnosis, Care is also taken that the patient is not taking antibiotics or had performed a barium enema; both will mask, any parasitic infection. Three stool samples should be examined as one sample every other day.
1. Macroscopic Examination
- The consistency should be noted whether formed, semi-formed or liquid, since trophic forms of protozoa are found in liquid or diarrheic stools, while cystic forms are found more in the formed and semi-formed samples.
- The composition of the sample is also important whether there is blood or mucus such as in dysentery.
- The colour is pale as in steatorrhea, It can also be black or red in colour when the iron is taken or when there is intestinal haemorrhage.
- The presence of adult parasites is also diagnostic.
2. Microscopic Examination
Direct Saline Smear
It is used to study the motility of protozoa if the stool sample is examined within one hour after discharge. Helminthic eggs may also be seen if they are present in large numbers.
Iodine Smear
In this method, stool samples are emulsified in a 1% iodine solution, The iodine stains the cysts of protozoa revealing their details. Its main disadvantage is that the trophozoites are rapidly killed by the iodine and therefore become non-motile.
The two above techniques are useful only when helminth eggs and protozoa are in large numbers. If they are scarce or scanty then concentration techniques of value. The concentration methods fall into two general classes: sedimentation and floatation methods.
Concentration Techniques
Sedimentation Methods
Principle: most helminthic eggs and protozoal cysts are heavier than suspending liquid so they sink and concentrate at the bottom of the tube.
- Saline Sedimentation.
- Formol Ether Concentration Technique: It includes centrifugation of the stool sample after the addition of ether and formalin. The ether adsorbs the faecal debris which becomes lighter than water and floats to the top and the addition of formalin fixes and preserves the specimens.
Floatation Method
Principle. This involves the use of solutions, which have higher specific gravity than the organisms to be floated so that the organisms rise to the top and the debris sinks to the bottom. The floatation procedure permits the separation of protozoan cysts and eggs of some helminths by using concentrated saline or zinc sulphate 33%, The main disadvantage is that heavy and operculated eggs do not concentrate well with this technique.
Permanent Stained Smears
These can be used identification intestinal protozoa.
Additional Techniques Used for Identification of Intestinal Parasites
Quantitative Estimation Helminthic Ova Stools
The correlation of egg production with adult worm burden is possible, Estimation of the intensity of infection of schistosomes as a test cure after treatment also important.
Kato Katz Technique
A portion of a sieved stool sample is transferred to a hole of the template on a slide, The eggs are then counted and the number per gram of faeces is calculated.
Adhesive Tape Technique (Cellotape)
This is used to diagnose parasitic infections by perianal swabs using the adhesive surface of cellophane tape, which is then placed on a microscopic slide. The examination is best done in the morning before bathing.
Urine Examination
1. Macroscopic Examination
The colour of urine can aid in the preliminary diagnosis of a parasitic infection.
- A white colour can denote chyluria and is suggestive of filarial infection. A drop of ether can dissolve the fat and the larva (microfilaria) can then be easily observed in the sediment.
- Smoky urine denotes the presence of blood and this can mask Schistosoma haematobium eggs. A drop of diluted acetic acid causes haemolysis of the red blood cells and the ova can then be seen.
2. Microscopic Examination
Urine is collected in a clean conical glass receptacle. The urine sample is concentrated either by centrifugation, or it is left for 15-20 minutes to allow any suspended material to sediment. With a pipette, a drop of the sediment is placed on a clean slide and examined microscopically.
3. Other Techniques
Membrane Filtration Technique: It is used for the concentration of eggs using a nucleopore filter, This test can be used to detect Schistosoma haematobium eggs in urine.
Aspirates
1. Duodenal Aspirate
Duodenal Capsule Technique (Enterotest)
This is a non-invasive method for sampling duodenal contents. The device consists of a length of nylon thread in a gelatin capsule. The thread protrudes through one end of the capsule and is taped to the side of the patient’s face, The capsule is swallowed, and the thread is retrieved 4 hours, Bile-stained mucus is scraped off in a petri-dish, which is then examined microscopically.
2. Bone Marrow Aspirate
The bone marrow is aspirated from the sternum in adults and the iliac crest in children.
children
3. Aspirates from Cutaneous Ulcers
Material aspirated should be from the edge of the ulcer not from the floor. It is important that the surface of the ulcer be thoroughly cleaned before specimens are taken as any contamination of material with bacteria or fungi may prevent organisms from growing culture, Part specimen taken should be placed on a side and stained with Giemsa. The other part should be put in a cultural medium.
Sputum
Sputum is examined to detect the diagnostic stages of parasites living in the lung, and the migratory stages of some parasites or parasites which result from the rupture of cysts or abscesses in the lungs. The appearance of the sputum is examined whether it is watery, purulent, or bloody or if it contains rusty brown particles.
If the sample shows rusty brown particles, an un-concentrated sample from these particles is examined first. To dissolve excess mucus, add an equal volume of 30g/L NaOH solution.
Biopsy Specimens
Tissue biopsy specimens are recommended for the diagnosis of a number of parasites as rectal and muscle biopsies. In cutaneous lesions e.g., leishmaniasis ulcers, skin scraping with microscopic analysis and punch biopsies with tissue-impression smears can also be diagnostic as the previously mentioned needle aspiration technique under the same conditions and precautions.
Blood Examination
Samples of blood can be taken from the fingertip, earlobe, or venipuncture. Blood from the fingertip and earlobe should be collected without an anticoagulant, while those from the vein should be collected in tubes with an anticoagulant such as EDTA.
1. Blood Films
Two types of blood films can be made: thin and thick blood films, Thick blood films have the advantage that a larger amount of blood is examined which increases the possibility of detecting light infections. However, thin blood films show morphological characteristics better.
2. Buffy Coat films
The buffy coat is the layer of white cells resulting from the centrifugation of whole citrated blood. A thin film is prepared from the cells of the buffy coat examined after staining with Giemsa.
3. Quantitative Buffy Coat Technique (QBC)
Microhaematocrit tubes are precoated with acridine orange, a fluorescent dye. Following centrifugation, beneath the buffy coat or white cell layer, there is a red cell layer that contains the acridine orange-stained parasites which fluoresce when viewed by a fluorescent microscope.
4. Knott’s Concentration Technique
This technique is based on concentration by centrifugation and the addition 2% formalin solution. The sediment will then be stained by Giemsa and examined microscopically.
You can follow science online on YouTube from this link: Science online
You can download Science Online application on Google Play from this link: Science online Apps on Google Play
Myiasis classification, diagnosis, causes, treatment & medicolegal importance
Importance & types of fungi, Opportunistic infections & Predisposing factors to candidosis
Vaccines types, Live vaccines, Inactivated vaccines, Subunit vaccines, Naked DNA & mRNA vaccines
Features and classification of viruses, Defective viruses & Viral vectors used for gene therapy