Pyrimidine metabolism, Orotic aciduria and Inhibition of pyrimidine synthesis (anti-cancerous drug)
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine, One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), Pyrimidine has nitrogen atoms at positions 1 and 3 in the ring, The other diazines are pyrazine (nitrogen atoms at the 1 and 4 positions) and pyridazine (nitrogen atoms at the 1 and 2 positions).
Pyrimidine
Pyrimidines are simple aromatic compounds composed of carbon and nitrogen atoms in a six-membered ring, The term pyrimidine is used to refer to pyrimidine derivatives, most of the three nitrogenous bases that, along with the two purines, are the building blocks of both deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), The pyrimidine nitrogenous bases are derived from the organic compound pyrimidine through the addition of various functional groups.
Pyrimidine biosynthesis
Sources of atoms for pyrimidine ring
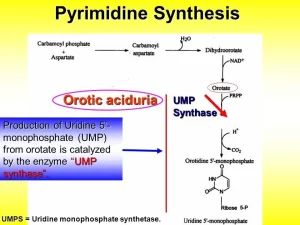
The sources of atoms of the pyrimidine ring are carbamoyl phosphate and aspartic acid. Carbamoyl phosphate is also a precursor of urea, but in the pyrimidine synthetic pathway, carbamoyl phosphate is made in the cytoplasm by carbamoyl phosphate synthetase II (CPS-II). Carbamoyl phosphate processed through the urea cycle is synthesized in the mitochondria by carbamoyl phosphate synthetase I (CPSI).
Steps of pyrimidine nucleotides biosynthesis
1. Pyrimidine biosynthesis begins with the formation of carbamoyl phosphate from glutamine, ATP and CO₂. This reaction is catalyzed by cytosolic carbamoyl phosphate synthetase (CPS II), Commited step in mammals including human. This enzyme differs from the mitochondrial carbamoyl phosphate synthetase (CPS I) used in urea synthesis. CPS I uses ammonia as the source of nitrogen, whereas CPS II uses the γ-amine group of glutamine. Unlike the purine ring, the pyrimidine ring is synthesized before being attached to ribose-5-phosphate, which is donated by PRPP.
2. Condensation of carbamoyl phosphate with aspartate forms carbamoyl aspartate in a reaction catalyzed by aspartate transcarbamoylase. This is the committed step. (Prokaryotes).
3. Ring closure via loss of water, catalyzed by dihydroorotase, forms dihydroorotic acid.
4. Abstraction of hydrogens from C5 and C6 by NAD+ introduce a double bond forming orotic acid, a reaction catalyzed by mitochondrial dihydroorotate dehydrogenase, All other enzymes of pyrimidine biosynthesis are cytosolic.
5. Transfer of a ribose phosphate moiety from PRPP forming orotidine monophosphate (OMP), which is catalyzed by orotate phosphoribosyl transferase.
6. Decarboxylation of orotidylate forms uridine monophosphate (UMP), the first true pyrimidine ribonucleotide.
7,8. Phosphate transfer from ATP yields UDP and UTP in reactions analogous to those for the phosphorylation of purine nucleotide monophosphates.
9. UTP is aminated to CTP by glutamine and ATP.
10. Reduction of ribonucleoside diphosphates (NDPs) to their corresponding deoxynucleoside diphosphates (dNDPs).
11. dUDP is dephosphorylated to DUMP.
12. Methylation of dUMP at C5 by N5, N10-methylene THF, catalyzed by thymidylate synthetase, forms thymidine monophosphate (TMP). The salvage pathway depends mainly on the phosphorylation of nucleosides by kinases and ATP.
Regulation of pyrimidine synthesis
The reaction catalyzed by CPSII is inhibited by UMP and stimulated by PRPP.
Inhibition of pyrimidine synthesis (anti-cancerous drug)
- Methotrexate: For further pyrimidine synthesis to occur, dihydrofolate must be reduced back to tetrahydrofolate, a reaction catalyzed by dihydrofolate reductase. Methotrexate inhibits dihydrofolate reductase so it interferes with pyrimidine (Thymine) synthesis that is needed by the highly dividing cells so it is used as an anti-cancerous drug.
- 5-florouracil is competitively inhibited thymidylate synthase thus interfering with TMP synthesis that is needed by the highly dividing cells so it is used as an anti-cancerous drug.
Pyrimidine catabolism
Catabolism of pyrimidine occurs mainly in the liver, The end products of pyrimidine catabolism are highly water-soluble: CO2, NH3, β-alanine and β-aminoisobutyrate.
Clinical disorders of pyrimidine metabolism
Orotic aciduria
It is a genetic disorder of pyrimidine metabolism caused by a deficiency of
- Orotate phosphoribosyl transferase.
- Orotodylic acid decarboxylase (OMP decarboxylase).
So, orotic acid becomes accumulated in the blood and is excreted in the urine.
Manifestations
- Growth retardation.
- Mental retardation.
- Anaemia.
- Excretion of large amounts of orotic acid and dihydroorotic acid in urine.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science online application on Google Play from this link: Science online Apps on Google Play
Importance of Nucleosides, Nucleotides, Purines, Pyrimidines & Sugars of nucleic acids
Gout causes, Steps of purine biosynthesis, Purine metabolism importance & disorders
Protein definition, structure, order, denaturation & Bonds responsible for protein structure
Protein structure, Classification, properties of amino acids & Formation of peptide bonds