Importance of Nucleosides, Nucleotides, Purines, Pyrimidines & Sugars of nucleic acids
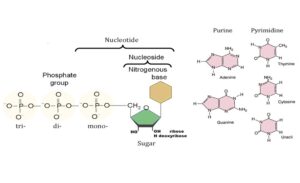
Nucleotides are the building blocks of nucleic acids. A nucleic acid is a polynucleotide chain that can be hydrolyzed by the enzymes nucleases. Each nucleotide can be hydrolysed by the enzyme nucleotidase into a nucleoside and a phosphoric acid. Each nucleoside can be hydorlysed by the enzyme nucleosidase into a nitrogenous base (pyrimidine or purine) and a pentose sugarpentose sugar (ribose or deoxyribose).
Nucleotide chemistry
Although the name of nucleic acids suggests their location inside the nuclei of cells, yet they are present both in the nuclei and cytoplasm of cells. Deoxyribonucleic acids (DNA) are present mainly in the nuclei while ribonucleic acids (RNA) are present mainly in the cytoplasm of cells.
Pyrimidines
Pyrimidine bases are formed of heterocyclic rings containing nitrogen, so they are called nitrogenous bases. In humans, there are 3 major pyrimidine bases found in the nucleotides forming the monomeric units of nucleic acids, they are:
- Uracil (2,4-dioxypyrimidine).
- Cytosine (2-oxy-4-amino pyrimidine).
- Thymine (2,4-dioxy-5-methylpyrimidine = 5-Methyluracil).
Uracil is found only in RNA, thymine in DNA, while cytosine is found in both RNA and DNA.
Purines
These are nitrogenous bases made of 2 fused rings, a pyrimidine and an imidazole ring. There are 2 major purine bases found in the nucleotides forming the monomeric precursors for both RNA and DNA in the living organisms including humans, they are:
- Adenine (6-amino purine).
- Guanine (2-amino-6-oxypurine)
The structure of the purine ring, the way of numbering the positions in the ring. In humans, a completely oxidised purine base, uric acid, is formed as the end product of purine catabolism.
Sugars of the nucleic acids
The nucleic acids are classified into two large groups according to the type of sugar they contain. One type of nucleic acids contains D-ribose, hence the name ribonucleic acid, or RNA. The second type of nucleic acids contains D-2-deoxyribose, hence the name deoxyribonucleic acid, or DNA. In nucleic acids, ribose or deoxyribose occur in the furanose ring structure and in the β-form.
Nucleosides
A nucleoside is composed of a purine or a pyrimidine base to which a sugar (ribose or deoxyribose) is attached. The sugar is present in the β-D configuration and is attached by its carbon No. 1 to N1 of pyrimidine or N9 of purine bases through N-glycosidic linkage.
Nucleotides
Nucleotides are nucleosides phosphorylated on one or more of the hydroxyl groups of the sugar ( ribose or deoxyribose). The position of the phosphate in the nucleotide is indicated by a numeral. For example, adenosine with phosphate attached to carbon 5 of the sugar ribose would be designated adenosine-5- phosphate.
If the phosphate is attached to carbon 3 of the ribose, the nucleotide would then be designed adenosine-3-phosphate. The prime mark after the numeral is required to differentiate position on the sugar moiety from the numbered position on a purine or pyrimidine base, which would not be followed by the prime mark.
A nucleotide of deoxyadenosine with phosphate moiety attached to the carbon 5 position of the sugar would be designed deoxyadenosine-5-phosphate. The abbreviations of nucleoside monophosphate e.g. AMP, GMP, CMP, …..etc. are given to the mononucleotides present in the free form i.e. not a component of the nucleic acid polynucleotide.
Naturally occurring nucleotides
In addition to the importance of nucleotides as components of nucleic acids, a number of nucleotides of great biological importance exist in the free state in living cells. These include the following:
Adenosine triphosphate (ATP) and adenosine diphosphate (ADP) and Guanosine triphosphate and diphosphate (GTP and GDP): are important in the collection and utilization of free energy in the living cells. ATP is the most abundant intracellular free nucleotide and is the source of high-energy phosphate for most energy-requiring reactions in the cell.
Cyclic AMP (cAMP=3´, 5´-adenosine monophosphate) and Cyclic GMP (cGMP = 3´, 5´- guanosine monophosphate): they serve as chemical mediators for hormonal action of many hormones.
S-adenosylmethionine is a nucleoside that is formed of adenine and ribose with methionine at 5´. It serves as a methyl donor in most of the transmethylation reactions in the body.
Uridine triphosphate (UTP): serves as a source of high energy phosphate for some reactions in the cell.
Structure of Cytoplasm, The function of centrosome & Cytoplasmic inclusions
The function of Cytoplasmic organelles, Mitochondria, Peroxisomes & Cytoskeleton
Cell Structure, the function of Golgi apparatus, Endosomes & Lysosomes
Nucleus components, function, diagram & classification of chromosomes
Cytoplasmic organelles, Ribosomes & Endoplasmic reticulum function, structure & definition
Regulation of the cell cycle, DNA synthesis phase, Interphase & Mitosis
Cell division types, Mitosis, Meiosis, Reductional division & Equatorial division
Cell proliferation, mechanisms of Cell Death, types & sources of stem cells