Gout causes, Steps of purine biosynthesis, Purine metabolism importance and disorders
Purines are essential for the synthesis of nucleic acids, proteins, and other metabolites as well as for energy-requiring reactions, Purines are the main components of nucleotides in cell proliferation, so, impaired purine metabolism is associated with the progression of cancer, purine nucleotides can be synthesized by the de novo and/or the so-called “salvage” pathways.
Purine metabolism
De novo purine nucleotides biosynthesis:
Purine nucleotides are important for all living organisms, they serve as precursors for the synthesis of nucleic acids and other metabolites. They also function as sources of energy for physiologic and metabolic reactions.
Sources of atoms in the purine ring
The atoms of the purine ring are contributed by a number of compounds, including the amino acids aspartic (N1), glycine (C4, C5. N7), and glutamine (N3, N9), CO₂ (C6), and derivatives of tetrahydro folic acid (C₂, C8). The liver is the major site for denovo synthesis of purine.
Steps of purine biosynthesis
The purine ring is constructed by a series of reactions that add the donated carbons and nitrogens to a preformed ribose 5-phosphate obtained from the hexose monophosphate shunt:
- The first step is the transfer of pyrophosphate from ATP to C1 of ribose 5-phosphate-forming 5-phosphoribosyl-1-pyrophosphate (PRPP). The enzyme used is 5-phosphoribosyl 1-pyrophosphate synthetase. PRPP is also an intermediate in NAD+ and NADP+ and pyrimidine nucleotide biosynthesis, and in the purine salvage pathway.
- Displacement of pyrophosphate from PRPP by the amide nitrogen of glutamine forms 5-phosphoribosylamine. The reaction involves inversion of configuration at C1, and forms the β-N-glycosidic bond. This step is catalyzed by the enzyme PRPP glutamylamidotransferase.
- After a series of reactions inosine monophosphate IMP is formed.
Conversion of IMP to AMP
The addition of aspartate to IMP forms adenylosuccinate using the enzyme adenylosuccinate synthetase, Release of fumarate forming adenosine 5 monophosphate (AMP) is catalyzed by adenylosuccinate
Conversion of IMP to GMP
Oxidation of IMP by NAD, catalyzed by IMP dehydrogenase forms xanthosine monophosphate (XMP). Transamidination by the amide nitrogen of glutamine forms guanosine monophosphate (GMP).
Conversion of AMP and GMP to their respective nucleoside di- and triphosphates involves successive phosphoryl transfer from ATP, catalyzed by nucleoside monophosphate kinase and nucleoside diphosphate kinase respectively.
Biosynthesis of deoxyribonucleotides:
Nucleotides synthesized by the pathways described are all ribonucleotides. These can be used as building blocks in RNA synthesis, The nucleotides required for DNA synthesis are 2′-deoxyribonucleotide, which are produced from ribonucleoside diphosphate, Deoxyribonucleotides contain 2-deoxyribose instead of ribose.
Deoxyribonucleotides are formed by direct reduction at 2-carbon in the ribose moiety of the corresponding nucleotide. The reduction at C2 carbon occurs only after the conversion of the nucleotide to its nucleoside diphosphate (ADP, GDP, UDP, CDP → (dADP, dGDP, dGDP, dUDP, dCDP, respectively), This reaction is catalyzed by the ribonucleotide reductase complex, Reduction requires thioredoxin (a protein cofactor), thioredoxin reductase (a flavoprotein), and NADPH.
Regulation of purine biosynthesis
- The major determinant of the overall rate of de novo purine nucleotide biosynthesis is the concentration of PRPP intracellularly, a parameter that reflects the relative rate of PRPP synthesis, utilization, and degradation, The rate of PRPP synthesis depends on both the availability of ribose-5-phosphate and on the activity of PRPP synthetase, an enzyme sensitive to the purine ribonucleotides that act as its allosteric regulators.
- PRPP amidotransferase is sensitive to feedback inhibition by purine nucleotides (AMP, GMP) PRPP concentration is a positive stimulator for amidotransferase, However, regulation of purine synthesis via the amidotransferase is probably of less physiologic importance than regulation of PRPP synthetase.
- Two mechanisms regulate the conversion of IMP to GMP and AMP AMP feedback regulates adenylosuccinate synthetase, and GMP feedback inhibits IMP dehydrogenase.
Inhibition of purine synthesis
1- Inhibiting the formation of tetrahydrofolate compounds can block purine synthesis, as carbons (C2, C8) are derived from N10 formyl and N5,10 methenyl tetrahydrofolate 2-6-mercaptopurine inhibits reactions adenylosuccinase and IMP dehydrogenase.
Purine salvage pathway
Definition: Reuse of Purines or to less extent their nucleosides that result from the normal turnover of cellular nucleic acids and are not degraded to be reconverted into nucleoside triphosphate and used by the body.
Significance:
- Require far less energy than does de novo synthesis.
- Provides tissues incapable of synthesizing purines de novo with their needs of nucleotides, for example: human brain has a low level of PRPP amidotransferase, also erythrocytes and polymorphonuclear leukocytes can’t synthesize 5-phosphoribosylamine.
Site: It occurs primarily in extrahepatic tissues.
Mechanism:
- The most important mechanism involves phosphoribosylation of a free purine base by PRPP, forming a purine 5′-mononucleotide. PRPP-dependent phosphoribosylation of purines is catalyzed by adenine phosphoribosyltransferase (converts adenine to AMP) and hypoxanthine-guanine phosphoribosyltransferase (converts hypoxanthine or guanine to IMP or GMP).
- A second salvage mechanism involves direct phosphorylation of purine ribonucleoside by ATP using kinase enzyme.
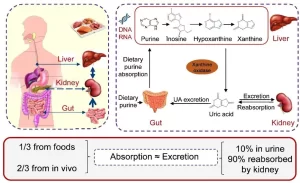
Purine catabolism
Uric acid is the end product of purine catabolism. Adenosine is first deaminated to inosine by adenosine deaminase, then phosphorolysis of N-glycosidic bonds of inosine and guanosine, catalyzed by purine nucleoside phosphorylase release ribose 1-phosphate and a purine base. Hypoxanthine and guanine next form xanthine in reactions catalyzed by xanthine oxidase and guanase, respectively. Xanthine is then oxidized to uric acid in a reaction catalyzed by xanthine oxidase.
Clinical disorders of purine metabolism
Hyperuricaemia
Definition: It is characterized by an excessive amount of uric acid in the blood (Normal blood level of uric acid is 3-7 mg/dL in males and 2-6 mg/dL in females.)
Causes; In many cases, it is due to accelerated production of uric acid, secondary to the degradation of unusually large quantities of purines.
Types
I- Primary hyperuricaemia may be due to:
a) Abnormalities of purine metabolism.
- PRPP-synthetase mutant forms of PRPP synthetase have been detected which are not subject to allosteric regulation by inorganic phosphate or to feed back inhibition. Under this condition, the intra-cellular concentration of PRPP is elevated leading to increased formation of phosphoribosylamine With the result that purines are overproduced. Overproduction in turn leads to increased catabolism and elevated levels of urate.
- Partial HGPRtase deficiency. A characteristic of this deficiency is the overproduction of purine nucleotide. This may be due to the lack of HGPRIate activity, leading to a decrease in the amount of hypoxanthine or guanine that can be salvaged Consequently the level of PRPP is increased because PRPP is not consumed via the salvage enzyme.
b) Abnormalities in other metabolic pathways.
- A deficiency in G-6-phosphatase (glycogen storage disease, type I, Von Gerk’s) leads to increase purine nucleotide de novo synthesis. The lack of conversion of glucose-6-phosphate to glucose leads to increased HMS activity. The increased utilization of G-6-P via the shunt results in increased R-5-P levels and consequently PRPP levels.
- An elevation of glutathione reductase activity has also been correlated with increased uric acid levels. Glutathione reductase generates NADP+, which is required to drive the first two reactions of HMS. This will lead to increased PRPP levels etc.
Il- Secondary hyperuricaemia is due to:
- Some diseases as in malignancy which enhance tissue turnover. Also in case of tissue breakdown (after treatment of large malignant tumors by radiotherapy or cytotoxic drugs)
- Also, hyperuricaemia can be due to a reduced rate of urate excretion
Gout
Definition: Gout is a metabolic disease characterized by an excessive amount of uric acid in the blood (hyperuricaemia).
Pathophysiology: serum urate levels exceed the solubility limit resulting in the crystallization of sodium urate in soft tissues and joints forming deposits, and causing an inflammatory reaction, especially in the big toe. Sodium urate may be precipitated in the kidney tubules causing renal stones.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science Online application on Google Play from this link: Science Online Apps on Google Play
Importance of Nucleosides, Nucleotides, Purines, Pyrimidines & Sugars of nucleic acids
Protein definition, structure, order, denaturation and Bonds responsible for protein structure
Protein structure, Classification, properties of amino acids & Formation of peptide bonds
Protein biosynthesis steps, site, importance, inhibitors & Protein maturation