Attempts of elements classification, Mendeleev, Moseley & Modern periodic table
The elements
The scientists classified the elements according to their properties to facilitate their study and to find a relationship between the elements, and their chemical and physical properties.
Mendeleev’s periodic table
There were only 67 elements that were known in Mendeleev’s periodic table, Mendeleev classified the elements in ascending order according to their atomic weights.
Mendeleev left gaps in his table predicting the discovery of the new elements, and he corrected the atomic weights of some elements which were estimated wrongly.
But Mendeleev had to make a disturbance in the ascending order of the atomic weights of some elements to put them in the groups that suit their properties.
Mendeleev had to deal with the isotopes of one element as different elements due to the difference in their atomic weights. So, He had to put more than one element in one cell of his table.
Moseley’s periodic table
Moseley classified the elements in ascending order according to their atomic numbers, He added the zero group which includes the inert gases to the table, and he specified a place below the table for lanthanides and actinides elements.
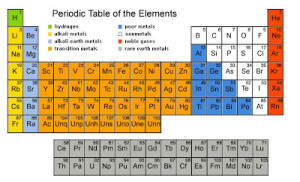
The modern periodic table
The elements are classified in ascending order according to their atomic numbers, and the way of filling the energy sublevels with the electrons, The number of the known elements in the modern periodic table till now is 116 elements, 92 elements are available in the Earth’s crust, while the rest are prepared artificially.
There are 7 horizontal periods, and 16 vertical groups (18 vertical columns), and the elements are classified into 4 blocks (s, p, d, and f).
The elements of the same group are similar in the number of the electrons in outermost energy level. So, they are similar in their chemical properties, and they are different in the number of energy levels occupied by the electrons.
The elements of the same period are different in the number of the outermost electrons. So, they differ in their chemical properties. and they are similar in the number of energy levels occupied by the electrons.
You can download Science online application on google play from this link: Science online Apps on Google play
Graduation of the properties of the elements in the modern periodic table
Classification of elements in Long-form periodic table, Ionization Energy and Oxidation numbers
The graduation of the electronegativity of the elements in the periodic table