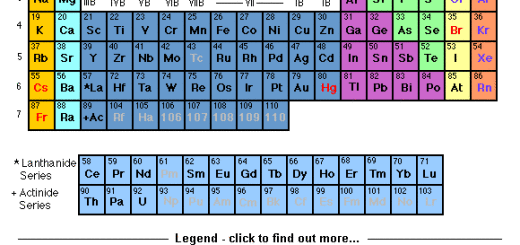
The description of the modern periodic table
The modern periodic table consists of 7 horizontal periods and 16 vertical groups (18 vertical columns). The elements of the modern periodic table are classified into 4 blocks (s, p, d, and f).
The periods in the modern periodic table
The periods include elements of different properties, and they have the same number of energy levels.
By increasing the atomic number for the elements of the period, the atomic size decreases, the electronegativity increases and the metallic property decreases till we reach the metalloid, then the nonmetallic property increases.
The groups in the modern periodic table
The groups include elements of similar properties, and they have the same number of electrons in the outermost energy level.
By increasing the atomic number of the elements in the groups, The atomic size increases, the electronegativity decreases, the metallic property increases in the groups that start with the metal, and the nonmetallic property decreases in the groups that start with the nonmetal.
The blocks in the modern periodic table
There are 4 blocks (s, p, d, and f). s-block elements are located on the left side of the periodic table and they are arranged in two groups (1 A) and (2 A).
p-block elements are located on the right side of the periodic table, and they are arranged in six groups (3 A), (4 A), (5 A), (6 A), (7 A), and the zero group.
d-block elements are located in the middle of the periodic table, and they are arranged in eight groups (3 B), (4 B), (5 B), (6 B), (7 B), (8), (1 B) and (2 B).
d-block elements are known as transition elements, They appear to start from the period (4), All the groups in d-block take the letter “B” except group (8) which consists of three vertical columns.
f-block elements are located below the periodic table, They include lanthanides and actinides.
The elements of the group (A) are located on the left and right sides of the table. The elements of group (B) are located in the middle of the table, and the new number of group (5 A) is (15), while that of the zero group is (18).
You can download the Science Online application on Google Play from this link: Science online Apps on Google Play
Elements of p-block, General properties of group 5A elements (group 15)
The most famous elements in 5A group (Nitrogen properties, Preparation & compounds)
Transition elements & Economic importance of elements of the first transition series
The lanthanides and actinides in the modern periodic table
Modern periodic table and classification of Elements
Graduation of the properties of the elements in the modern periodic table