The importance and properties of the bases (alkalis)
The bases (alkalis)
The bases are the substances that dissociate in the water producing the negative hydroxide ions (OH–), Examples of some bases such as sodium hydroxide (the caustic soda) NaOH, calcium hydroxide (lime water), and potassium hydroxide (KOH).
The properties of the bases (alkalis)
The bases change the colour of litmus paper into blue due to the presence of the negative hydroxide ions (OH–), The aqueous solutions of the bases have a bitter taste and the bases destroy the chemical properties of the acids.
The bases feel slippery and soapy as they dissolve the fatty acids and oils from your skin, the bases are involved in the production of soap, The bases which are soluble in the water are called the alkalis, and the chemical formula of all alkalis ends with (OH–).
The bases conduct an electric current, This is a common property shared with the salts, the acids, and the bases, The salts are grouped together into a category called electrolytes, The bases react with oils and grease to form soap molecules and they have the tendency to corrode metal surfaces.
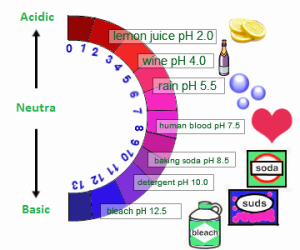
The strong bases can burn your skin, so you should not touch the bases and the acids with your bare hand as they have a corrosive effect on the skin, The bases are good conductors of the electricity and they show a pH value of more than 7.
The reaction between a base and a metal form the salt and release hydrogen gas, and this reaction can only occur when a metal is strong enough to displace another metal from its parent constituent.
The types of bases
There are strong bases (They are completely ionized in water to produce hydroxide ions) and the weak bases (They are partially ionized and the equilibrium lies mostly towards the reactants’ side.
Types of compounds, Properties of Acids, Bases (alkalis), Oxides and Salts
Classifications of bases according to strength (degree of ionization) & molecular structure
Properties of Acids and Bases & Theories defining acids and bases
Classification of Acids according to its strength (degree of ionization), Its source & Basicity
Elements of s-block, Properties of the first group elements 1A (Alkali metals) in the periodic table

I think it is ok