Properties of fluids, Factors affecting density and pressure
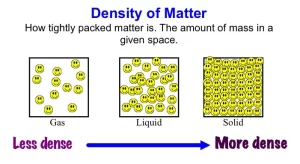
Matter can be found in nature in one of three states which are solid, liquid, and gas, Solid materials (like wood and glass) have a definite shape and volume, while liquids and gases (like water and air) have no definite shape but they take the shape of their container, so, they are called fluids.
Fluids
Fluids are materials that can flow and have indefinite shapes, There are two types of fluids:
- Liquids are characterized by: definite volume, smooth flow, and being incompressible.
- Gases are characterized by: occupying any space, taking the volume of their container, and can be easily compressed.
Properties of fluids
We will explain in details some of the physical properties characterizing the fluids which are density and pressure.
Density
Density is the mass of the unit volume of the substance or the mass of the body divided by its volume, The density of a material is given by the relation:
ρ = m / Vol
Where: m is the mass of the substance and measured in kg, Vol is the volume of the substance and measured in m3, consequently, the density is measured in (kg/ m3), When the density of iron = 7900 kg / m3, It means that the mass of 1 m3 of iron = 7900 kg.
When mixing two or more materials then: m (mix) = m1 + m2 + ……
ρ Vol = ρ1 (Vol)1 + ρ1 (Vol)2 + ……
Vol (mix) = (Vol)1 + (Vol)2 + ……
And if the mixture is diminished: Vol (mix) = [ (Vol)1 + (Vol)2 ] − Δ Vol
The factors that affect the density
Density differs from one material to another because of the differences in:
- The atomic weight of the element or the molecular weight of the compound.
- The distance between atoms (Interatomic distances) or molecules (Intermolecular spaces).
Density is considered a characteristic property of the material, because it is constant for the same material and does not change as the mass or volume of the material changes at the same temperature, It changes by changing the type of material or changing the temperature because the increase in temperature changes the intermolecular spaces between atoms or molecules and consequently the density.
Applications of density
Indicating how well the battery of the car is charged by measuring the density of the electrolytic solution inside it, When the battery is discharged, the density of its electrolytic solution (diluted sulphuric acid) decreases because of the chemical reaction with the lead plates and the formation of lead sulphate, When the battery is recharged, the sulphate is separated from lead plates and go back to the electrolyte and the density increases again.
Diagnosis of some diseases like Anemia by measuring blood density, The normal rate of blood density ranges from 1040 kg/m3 to 1060 kg/m3, if blood density exceeds 1060 kg/m3, this indicates an increase in the concentration of the red blood cells, Blood density preceded 1040 kg/m3, this indicates a decrease in the concentration of the red blood cells which indicates Anemia.
The increase of salt concentration in urine, By measuring the urine density, The normal density of urine is 1020 kg/m3 and some diseases cause an increase of salts in the urine that increase its density.
Relative density is the ratio between the density of a material to the density of water at the same temperature or it is the ratio between the mass of a certain volume of a material to the mass of the same volume of water at the same temperature.
The relative density of a substance can be determined from the relations:
Relative density of a substance = Density of material at a certain temperature / Density of water at the same temperature
Relative density of a substance = Mass of a certain volume of a material at a certain temperature/Mass of the same volume of water at the same temperature
The relative density is dimensionless because it is a ratio between two similar quantities, when the relative density of gasoline is 0.9, It means that the ratio between the density of gasoline to that of water at the same temperature = 0.9.
The density of a material can be determined by knowing its relative density using the following relation:
ρmaterial = ρrelaive × ρwater = ρrelaive× 1000
(Where: ρwater = 1000 kg / m3)
Pressure
When a force (F) acts on a surface of area (A), pressure (P) is produced on this area, The pressure at a point is the average force acting perpendicularly on the unit area surrounding this point.
Force perpendicular to the surface, so, P = F/A, mg/A
Force making angle θ with the surface, so, P = F sin θ / A
Force making angle θ with the normal to the surface, then, P = F cos θ / A
Where: Force (F) is measured in Newton (N) and area (A) is measured in m2, Thus, pressure is measured in N/m2 (Pascal) and its equivalent units are kg/m.s2 or J/m3.
When the pressure at a point = 500 N/m2, It means that the average force acting perpendicularly on the unit area surrounding this point = 500 N.
Factors affecting the pressure at a point:
- Average force acting perpendicular (F), (directly proportional) P ∝ F at constant A.
- Area surrounding this point (A), Inversely proportional P∝ (1/A) at constant F.
It is clear that as the area increases, the pressure decreases, so, wide tires are used in the heavy trucks, thus, the pressure due to the weight of the truck decreases on the road, So, tires do not sink in the sandy roads.
As the area decreases, the pressure increases, So, needles and pins have sharp tips, Thus, higher pressure is produced, so, they penetrate bodies easily.
Applications on the pressure
- Measuring blood pressure
- Measuring the air pressure inside the tires of a car
Measuring blood pressure: A normal person has two values for blood pressure (the contracting pressure and the relaxing pressure), it is said that the person is a blood pressure patient if one of these values has changed.
The systolic (contracting) pressure is the maximum value for the blood pressure when the heart muscle contracts and equals 120 torr for a normal person.
The diastolic (relaxing) pressure is the minimum value for the blood pressure when the heart muscle relaxes and equals 80 torr for a normal person.
When the blood pressure for a normal person is 120/80, It means that the maximum value for blood pressure in the artery when the heart muscle contracts is 120 torr, and the minimum value for blood pressure in the artery when the heart muscle relaxes is 80 torr.
Measuring the air pressure inside the tires of a car: The tire of a car is filled with air under a suitable high pressure so that the tangent area between the tire and the road is minimum and consequently the friction decreases which in turn decreases the hot temperature of the tire and vice versa.
Elephant foot pressure or man’s?
Pressure due to a pointed high heel is greater than that due to an elephant’s foot on the ground because the pressure is inversely proportional to the surface area.
Pressure at a point inside a liquid
When a liquid is put in a container, every point inside the liquid is affected by the weight of the liquid column which is its height (h), and the area of its base (A) which causes pressure at this point.
Pressure at a point inside a liquid is the weight of the liquid column which its base is the unit area surrounding this point and its height is the vertical distance from this point to the surface of the liquid.
When the pressure of a liquid at a point inside it = 2 ×106 N/m2, It means that the weight of the liquid column which is its base is the unit area surrounding this point and its height is the vertical distance between this point and the liquid surface = 2 ×106 N.
Deduction of the pressure value at a point inside a liquid
Imagine plate (X) of area (A) at a depth (h) inside a liquid of density ρ, This plate acts as the base of a column of the liquid, and the force acting on the plate (X) is the weight of the liquid column whose height is (h) and whose cross-section area is (A).
The weight of the liquid column (Fg) is determined by the relation:
Fg = mg
Where ( m ) is the mass of the liquid column.
m = ρ Vol
Vol = A h ∴ Fg = Ahρg
P = Fg / A = Ahρg / A
∴ P = ρgh
If the liquid surface is open to air then the total pressure at this point: P = Pa + ρgh, Where Pa is the atmospheric pressure, The pressure on a body at the bottom of a liquid is perpendicular on each point of its surface.
Factors affecting the pressure at a point inside the liquid:
- Density of the liquid (ρ), directly proportional, P ∝ ρ at constant g and h.
- Acceleration due to gravity (g), directly proportional, P ∝ g at constant ρ and h, g changes slightly from one place to another.
- Point depth (h), is directly proportional.
It is clear that as the depth (h) increases, the pressure (P) increases where P ∝ h, That is why the base of the dam must be thicker than its top to withstand the increase in pressure at the high depth, When the depth of points below the surface is same and so is the density (ρ), the pressures becomes the same where p = ρgh.
All the points at the same horizontal level inside the liquid have the same pressure, that’s why open seas and oceans have one horizontal surface of water, The pressure at a point inside the liquid is a scalar quantity.
Applications on the pressure at a point (Connected vessels, U-shaped tube and Mercuric barometer)