What are the types and properties of salts?
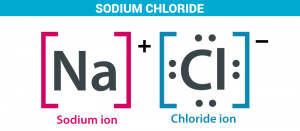
Salts are ionic compounds that result from the neutralization reaction of an acid and a base, they are composed due to the combination of a positive ion (or a positive atomic group) with a negative atomic group (or a negative nonmetal ion except oxygen).
Salts
Salts are composed of related numbers of cations (positively charged ions) and anions (negative ions) so that the product is electrically neutral (without a net charge), and these component ions can be inorganic.
Salts are produced from the combination of the positive ion with negative ion such as sodium chloride (the table salt) NaCl, and lead bromide.
Salts are produced from the combination of a positive ion with a negative atomic group such as sodium nitrate, magnesium carbonate, and unhydrous copper sulphate.
Salts are produced from the combination of a positive atomic group with negative ion such as ammonium chloride and ammonium bromide.
Salts are produced from the combination of positive atomic group with negative atomic group such as ammonium carbonate.
Salts are existed in the Earth’s crust or dissolved in the water, sodium chloride (NaCl) is the most important chemical compound for the human body, We get the salts from the sea, the blood has the similar chemical balance of sodium, potassium, calcium in our body.
Physical properties of salts
Salts exist in many different colors, sodium chromate is yellow, cobalt nitrate is red, potassium dichromate is orange, potassium permanganate is purple, nickel chloride hexahydrate is green, nickel chloride hexahydrate is blue, and sodium chloride is colorless or it may appear white when powdered or in small pieces.
The salt taste of seawater is due to the number present of salts such as sodium chloride and magnesium bromide, there is a lot of a variety of salts there in the saltwater.
Different salts can be in five basic tastes, The salt is salty such as sodium chloride, The salt is sweet such as lead diacetate which will cause lead poisoning if ingested, the salt is sour such as potassium bitartrate, It is bitter such as magnesium sulfate, and it is umami or savory such as monosodium glutamate.
Salts of strong acids and strong bases (the strong salts) are non-volatile and odorless, whereas the salts of either weak acids or weak bases (the weak salts) may smell after the conjugate acid such as the acetates like acetic acid (vinegar), and the cyanides like hydrogen cyanide (almonds) or the conjugate base such as ammonium salts like ammonia of the component ions.
Some mineral salts are classified into the salts dissolved in the water and the salts undissolved in the water, all salts of sodium, potassium, and ammonium are soluble in water such as all nitrates and many sulfates, barium sulfate, calcium sulfate (sparingly soluble).
Salts dissolved in the water such as sodium chloride, potassium sulphate, calcium nitrate, and sodium sulphide, The ions that bind tightly to each other and form highly stable lattices are less soluble because it is harder for these structures to break apart for the compounds to dissolve.
Most carbonate salts are not soluble in the water such as lead carbonate and barium carbonate, and soluble carbonate salts such as sodium carbonate, potassium carbonate, and ammonium carbonate.
Salts insoluble in the water such as silver chloride, lead iodide, and lead sulphate, The solid salts do not conduct electricity while the liquid salts do, and the solutions of salts also conduct the electricity.
Molten salts and solutions containing dissolved salts are called electrolytes such as (sodium chloride in the water) because they are able to conduct the electricity.
Economic importance of some common acids, bases and salts (minerals)
Salts formation methods & types of aqueous solutions of salts
Salts formation, The chemical formula of salts and its naming