The movement of electrons around the nucleus and the energy levels
The movement of electrons
The electrons are negatively (- ve) charged particles, They revolve around the nucleus with very high speed, The electron has a negligible mass relative to that of the proton or the neutron, so the mass of the atom is concentrated in the nucleus.
The number of the negative electrons which revolve around the nucleus is equal to the number of positive protons in the nucleus, so the atom is electrically neutral in its ordinary state.
The atomic number = the number of the protons = the number of the electrons.
The mass number = the number of the protons (the atomic number) + the number of neutrons.
What are the energy levels?
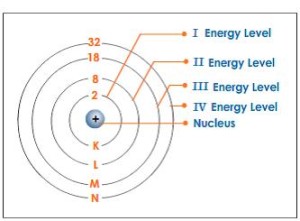
The electrons revolve around the nucleus in a number of orbits called the energy levels, and during their movement, they seem to be as a cloud around the nucleus, The energy levels are the imaginary regions around the nucleus in which the electrons move according to their energies.
The nucleus contains the protons and the neutrons, the first energy level can hold a maximum 2 electrons in one orbital, the second level can hold a maximum of 8 electrons that total distributed over 4 different orbitals, and the third energy level can hold a maximum of 18 electrons distributed over 9 different orbitals.
The maximum number of energy levels is seven levels (in the heaviest atoms) which are arranged from the nucleus according to their energies.
Each level has a certain amount of energy increases by the increase of the distance from the nucleus, the first energy level (K) has the least energy followed by the second level (L) and so on.
The seventh energy level (Q) has the highest energy, and the energy of level (L) is greater than that of level (K), The energy of the electron depends on the energy of the level which it revolves in, where the energy of the electron = the energy of the level.
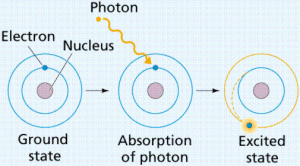
The electron is not transferred from one level to a higher one unless it gains some energy called the quantum which equals the difference between the energies of two levels and the atom will be excited atom.
The electrons lose this energy, it returns to its original level and the state of the atom is the ground state.
The excited atom is the atom that gains a quantum of energy, and the quantum is the amount of energy lost or gained by an electron when it transfers from one energy level to another.
Theories explaining the covalent bond, Octet rule & Overlapped orbitals concept
The quantum numbers and principles of distributing electrons
Chemical combination, Types of bonds (Chemical bonds & Physical bonds)