Classification of metals according to their chemical activity and What is the chemical activity of metals?
Metals can be classified according to their chemical activity into three groups which are very active metals, less active metals, and inactive metals. The high reactivity of active metals makes them useful in a variety of applications. Sodium is used in the production of chlorine and soap. Potassium is an essential nutrient for plants and animals.
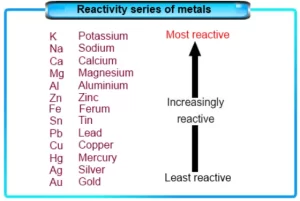
Very active metals
Very active metals are metals that react with oxygen as soon as being exposed to humid air, so they lose their metallic luster such as sodium and potassium, Sodium and potassium are kept under the kerosene surface to prevent their reaction with the atmospheric oxygen.
Less active metals
Less active metals are the metals that react with oxygen if they are left in the air for some days and a layer of rust is formed such as iron, aluminum, and copper.
Steel bridges and the holders of light bulbs are painted from time to time to protect them from rust and corrosion.
Metallic spare parts of cars are covered with grease to protect them from rust and corrosion, Washing of cooking pans that are made of aluminum with a rough material to remove any layer formed on them.
Inactive metals
Inactive metals are metals that find great difficulty in reacting with oxygen such as silver, platinum, nickel, gold, and chromium.
Silver and gold are used in making jewels because silver and gold are chemically poorly active.
Nickel, gold, and silver are used to cover other substances that rapidly gain rust to protect them from corrosion.
What is the classification of metals based on the reactivity series?
Metals can be classified into several categories based on their chemical activity, with the most common system using four main groups:
1. Most Active Metals
These are the most reactive metals and readily lose electrons to form cations. They are found in Groups IA (alkali metals) and IIA (some alkaline earth metals) of the periodic table. Examples include lithium (Li), sodium (Na), potassium (K), magnesium (Mg), and calcium (Ca). These metals react vigorously with water at room temperature, releasing hydrogen gas and forming a metal hydroxide solution. Due to their high reactivity, they are not found in their elemental state in nature but are bonded with other elements in minerals.
2. Moderately Active Metals
These metals are less reactive than the most active group. They don’t react with water at room temperature but can react readily with acids. They are located further to the right in Groups IA and IIA and some transition metals. Examples include zinc (Zn), iron (Fe), and lead (Pb). These metals require stronger acids or specific conditions to react and form salts.
3. Less Active Metals
This group includes metals with lower reactivity. They only react with strong acids or require high temperatures. They are found scattered across the periodic table, including some transition metals and metalloids. Examples include chromium (Cr), nickel (Ni), and tin (Sn). These metals require concentrated acids or specific oxidizing agents to participate in reactions.
4. Noble Metals
These are the least reactive metals. They are referred to as noble metals because they resist corrosion and oxidation due to their strong electron configuration. They are located on the far right of the periodic table, including platinum (Pt), gold (Au), and silver (Ag). Noble metals do not react with water or most acids at room temperature.
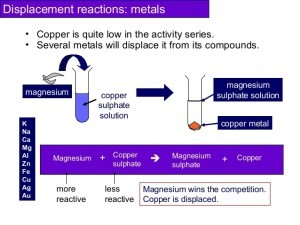
Active metals
Active metals are those that readily react with other substances due to their electronic configuration. They tend to lose electrons easily to form positively charged ions (cations). These metals are found on the leftmost side of the periodic table, in Groups IA and IIA.
The most active metals are the alkali metals in Group IA: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). These elements all have just one valence electron in their outermost shell, which they are very willing to give up. This makes them highly reactive, especially with non-metals such as halogens (like chlorine) and oxygen. Lithium is used in batteries and medications.
Alkali metals react vigorously with water to produce hydrogen gas and a metal hydroxide. The reactivity increases as you go down the group because the atoms get larger and it becomes easier to remove the outer electron. The alkali metals are so reactive that they are not found in nature in their elemental state, but rather combined with other elements in minerals. Francium is so reactive that it is radioactive and only exists in minute quantities in nature.
Some of the alkaline earth metals in Group IIA, such as calcium (Ca), strontium (Sr), and barium (Ba), are also considered active metals. They have two valence electrons, but they are also relatively loosely held and can be readily lost to form a +2 cation, although they are not quite as reactive as the alkali metals.
The reactivity of active metals increases as you go down their group in the periodic table. This is because the outer electrons are farther from the nucleus and therefore less attracted to it, making them easier to remove.
Properties of active metals
- Low ionization energy: They require very little energy to remove an electron from their outermost shell.
- High reactivity: They readily react with non-metals, such as halogens (e.g., chlorine, fluorine) and oxygen, to form ionic compounds. For example, sodium reacts vigorously with water to form hydrogen gas and sodium hydroxide.
- Softness: They are generally soft and can be easily cut with a knife. They are easily bent and shaped because the metallic bonds between the atoms are weak.
- Low melting and boiling points: They tend to have low melting and boiling points because the metallic bonds between the atoms are weak. The weak metallic bonds also make them easy to melt and vaporize.
- Good conductors of heat and electricity: They are good conductors because they have freely moving valence electrons.
- Due to their high reactivity, active metals are not typically found in nature in their pure form. They are usually found in compounds, such as oxides, sulfides, or carbonates.
Modern periodic table and classification of Elements
Radius property, Ionization potential, Electron affinity and Electronegativity
Metallic and nonmetallic properties, Acidic and basic properties in the periodic table
Chemical combination, Properties of Metals, Nonmetals and Noble (inert) gases