Enzymes types, function, Composition of Saliva and Properties of salivary amylase.
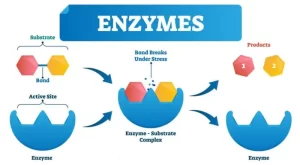
Enzymes help speed up chemical reactions in our bodies, they are essential for digestion, liver function, and much more, Too much or too little of a certain enzyme can cause health problems, Enzymes in our blood can help healthcare providers check for injuries and diseases.
What are enzymes?
Enzymes are organic compounds, proteins in nature, synthesized in living cells to accelerate the rate of biological reactions. Enzymes help speed up metabolism, or the chemical reactions in our bodies, They build some substances and break others down, All living things have enzymes. Our bodies naturally produce enzymes, But enzymes are also in manufactured products and food.
What do enzymes do?
Enzymes help in digestion, Digestion is the process of turning the food we eat into energy, there are enzymes in our saliva, pancreas, intestines, and stomach, They break down fats, proteins, and carbohydrates. Enzymes use these nutrients for growth and cell repair, Enzymes help with Breathing, Building muscle, Nerve function, and Ridding our bodies of toxins.
Saliva
Saliva is secreted in the oral cavity by three pairs of salivary glands: parotid, submaxillary, and sublingual. It is a colorless viscous fluid. The volume secreted by 24 hours is about one liter. Its reaction is variable but generally with a pH value 6-7, mostly neutral or slightly acidic. It has a specific gravity of 1.002-1.008.
Composition of Saliva
- 99.5%: water.
- 0.5%: solids, in the form of:
- 0.3%: organic substances, e.g. mucin, enzymes (amylase), urea, glucose, and lactic acid.
- 0.2%: inorganic substances, e.g. Na+, K+, Ca+2, Cl−, HCO3, PO4−3, SCN− and SO4+2.
Salivary Amylase enzyme (Ptyalin)
Properties of salivary amylase
- Salivary amylase enzyme is a hydrolase (α-1,4 glucosidase) that hydrolyses the α-1,4 glucosidic bonds of starch leading to the formation of dextrins and maltose.
- Protein in nature as all enzymes.
- Optimum temperature: 37°C.
- Optimum pH: 6.8
- Activators: halogen ions especially Cl− ions.
- Duration of action: 15 minutes and stopped by gastric acidity.
Steps of hydrolysis of starch amylase
- Starch → amylodextrin + maltose
- Amylodextrin → erythrodextrin +maltose
- Erythrodextrin → achrodextrin + maltose
The term amylo, erythro and achro indicate the color of the dextrin with iodine i.e. violet, red and yellow colors respectively.
Experiments performed on salivary amylase
For the following experiments on salivary amylase, saliva is first obtained in a test tube, then it is diluted four times by mixing one volume of saliva with three volumes of water, or better with saline because this enzyme is activated by the presence of Cl− ions.
Determination of achromic point
The achromic point is defined as the time taken by salivary amylase to hydrolyse starch into achrodextrin at optimum conditions.
Steps:
- Prepare water bath at 37°C.
- Prepare a porcelain plate and add a drop of iodine into each well.
- To 3 ml of 1% starch solution in a test tube add an equal volume of solution containing salivary amylase, mix well, and place the tube immediately in the water bath. Record the time and take a drop of the mixture of the tube by a glass rod and mix it with the iodine drop present in the first well of the porcelain plate, the color will become blue.
- At half-minute intervals, take one drop of the starch-saline mixture and mix it with the iodine present in the other wells of the porcelain plate. The color, which was blue in the first well, will change to violet, then reddish, and finally yellow (i.e., no changes in the color of the iodine solution) in the next wells.
- Record the exact time taken for the color to be changed from blue to just yellow (which equals the time taken by starch to be changed to achrodextrin). This time represents the achromic point.
Effect of Temperature on Salivary Amylase Activity
1. Prepare three test tubes, in each place 3 ml of 1% starch solution + 3 ml of diluted saliva, and mix well.
2. Put the tubes immediately in the following temperatures.
- Tube No. 1: At 37°C water bath.
- Tube No.2: at room temperature (leave in the test-tube rock).
- Tube No.3: at boiling water bath.
3. Leave the tubes for the achromic point time.
4. Cool the tubes under running tap water.
5. Add 2 drops of iodine to each of the three tubes. It will be found that iodine gives different colors in three tubes, depending on the products of digestion of starch in each.
6. The expected colors may be as follows:
- Tube No.1: Yellow (achrodextrin is the product of starch digestion, salivary amylase is active, and 37°C is optimum for its maximal activity).
- Tube No.2: Reddish or violet (erythrodextrin or amylodextrin may be the product of the starch digestion which is incomplete by the end of the achromic point time. Room temperature is not optimum for the enzyme activity).
- Tube No.3: Blue (starch is not digested, salivary amylase is inactive, and boiling causes denaturation of the enzyme).
If Fehling test is performed on the products of starch digestion in each of the three tubes, the reduction obtained will be parallel with the coloration given by iodine, so one may expect strong reduction in the first tube, weak in the second and no reduction in the third.
Effect of pH on Salivary Amylase Activity
1. Prepare three test tubes, in each place 2 ml of 1% starch solution.
2. Add 2 ml of buffer solutions of different pH as follows:
- Tube No. 1: 2ml of buffer solution of pH 4.5.
- Tube No. 2: 2ml of buffer solution of pH 6.8.
- Tube No.3: 2ml of buffer solution of pH 13.
3. Add 4 ml of diluted saliva into each of the three tubes, and mix.
4. Put 37°C, leave for the achromic point time.
5. Then remove the tubes from the water bath, cool them under running tap water, and add 2-3 drops of diluted acetic acid to tube No.3.
6. Add 2 drops of iodine to each of the three tubes and notice the color changes in each tube. The expected colors may be as follows:
- Tube No. 1: Reddish or violet (erthrodextrin or amylodextrin may be the product of starch digestion which is incomplete by the end of achromic point time, pH 4.5 is not suitable for maximal enzyme activity).
- Tube No.2: Yellow (achrodextrin is the product of starch digestion, salivary amylase is active and pH 6.8 is optimum for its maximal activity).
- Tube No.3: Blue (starch is not digested, salivary amylase is inactive and pH 13 is not suitable for its activity).
The addition of acetic acid in tube No.3 will prevent the change of iodine to iodide under the effect of the alkaline pH 13. Iodide gives no color with starch while iodine gives a blue color with starch.
If the Fehling test is performed on the products of starch digestion in each of the three tubes, the reduction obtained will be parallel with the coloration given by iodine, so one may expect a weak reduction in the first tube, strong in the second and no reduction in the third.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download the Science Online application on Google Play from this link: Science Online Apps on Google Play
Enzymes meaning, function, examples & Mechanism of enzyme action
Isoenzymes structure, Coenzymes function & Classification of enzymes
Factors affecting the rate of enzyme reaction & Importance of enzyme inhibitors
Git function, Types of salivary secretion & Composition of saliva
Factors affecting the rate of enzyme reaction & Importance of enzyme inhibitors