Protein metabolism, Fate of absorbed amino acids and Biological importance of transamination
Protein is important for keeping the muscles, bones, and tissues healthy. Protein plays a role in many bodily processes, such as blood clotting, fluid balance, immune system responses, vision, hormones, and enzymes. Protein is very important for growth and development, especially during childhood, adolescence, and pregnancy, Both animal and plant foods can be excellent sources of protein.
Protein metabolism
Proteins are polymers of amino acids. They perform many essential functions in the mammalian body.
Sources of dietary proteins
- Animal: as milk, fish, meat, and eggs.
- Plant: as cereals and beans.
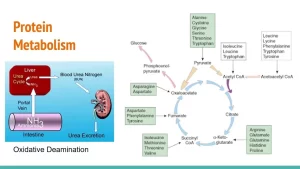
Fate of absorbed amino acids
Most of the absorbed amino acids pass to the liver through portal blood circulation. A small part escapes the liver to systemic circulation. This is responsible for an increase of blood amino acid level from 2-4 mgldL in a fasting state to 6-8 mgldL after a protein meal. Amino acids share anabolically and catabolically in the formation of some organic compounds as follows.
Anabolic pathway
Amino acids enter in the formation of tissue proteins for wear and tear, plasma proteins, hemoglobin, enzymes, and some hormones and also enter in the formation of non-protein nitrogenous compounds (NPN) as purines, pyrimidines, creatine, and thyroxine.
Catabolic pathway
- Urea: formed in the liver, is considered the main metabolic endoproduct of protein catabolism.
- Supplying energy: One gram of protein yields 4.1Kcal, only if there is a shortage in carbohydrates and fats.
Nitrogen Balance
There is no storage (depot) for protein, but there is a certain percentage of protein that undergoes a constant process of breakdown and resynthesis i.e. turnover. This is a normal process and is an essential feature of what is called “nitrogen balance”.
- Nitrogen balance is the difference between the total nitrogen intake (mainly in the form of dietary protein) and the total nitrogen loss (mainly in the form of undigested protein in stool and urea and ammonia in the urine). Also, nitrogen output is through nails, hair, and desquamated skin.
- Nitrogen equilibrium: The normal adult subject will be in nitrogen equilibrium when losses (in urine, feces, and sweat) just balanced by diet intake.
- Positive nitrogen balance: A condition in which there is an increase in the N₂ intake over the output. It may occur in growth, pregnancy, or convalescence from diseases.
- Negative nitrogen balance: A condition in which there is either decreased N₂ intake as in: starvation, poverty malnutrition, maldigestion, malabsorption, severe vomiting, severe diarrhea, or increased N₂ output as in hemorrhage, burns, old age, or debilitating disease.
General metabolism of proteins
The major pathway for amino acids excess after protein synthesis is the removal of the amino group and its conversion to ammonia to be excreted mainly in the form of urea. The liver is the major site of removal of the amino group from amino acids. The amino group is removed by different mechanisms: transamination oxidative deamination, non-oxidative deamination, and transamination.
I. Transamination
Its enzymes are termed transaminases, It transfers the amino group from an amino acid to a -keto acid, All the amino acids participate in the reaction of transamination except (threonine) and lysine) Vitamin Be is required as a coenzyme. Most transaminases require a -keto glutarate and glutamate as one of the reacting pairs. Transaminases are cytosolic and mitochondrial enzymes. It is a freely reversible process.
The most important transaminases are:
a) Aspartate transaminase (AST) or (GOT).
Aspartate transaminase catalyzes the transfer of an amino group from aspartate, It is an enzyme normally found in liver cells and to a lesser extent in cardiac muscles In the serum, it is considered a non-functioning enzyme as its level varies from 5-40 IU/L. Its increase in serum indicates either liver cell damage as in (viral hepatitis or muscle damage as in myocardial infarction
b) Alanine transaminase (ALT) or (GPT)
Alanine transaminase catalyzes the transfer of an amino group from alanine, This enzyme is normally found in liver cells, It is a non-functional serum enzyme, i.e. it is not normally found in plasma and does not perform any function in plasma, Its level in serum varies between 5 and 45 IU/L, Its increase indicates liver cell damage as in viral hepatitis.
Biological importance of transamination:
- Synthesis of new non-essential amino acids.
- Degradation of most amino acids except lysine and threonine.
- Formation of components of the citric acid cycle (filling up the reaction of the citric acid cycle).
- Transaminase enzymes are used in the diagnosis and prognosis of some diseases.
II. Oxidative deamination
It is catalyzed by:
1. Amino acid oxidases
Occur in the liver and kidney. It includes the removal of hydrogen (oxidation) and the removal of NH3 (deamination). There are D- and L-amino acid oxidases that oxidizes D-and L-amino acids respectively, to the corresponding a-keto acids, and the amino group is released as ammonia (NH3).
Molecular oxygen will react with reduced flavin giving H2O2 that is decomposed by catalase enzyme to H20 and oxygen. D-amino acid oxidase uses FAD as a coenzyme which is of limited natural occurrence in mammals and of high activity, whereas L-amino acid oxidase uses FMN as a coenzyme which is of natural occurrence in mammals, but of low activity. It properly functions in the deamination of lysine.
2. L-Glutamate dehydrogenase
The reaction is both mitochondrial and cytoplasmic, occurs mainly in the liver and kidney, ATP and GTP are allosteric inhibitors while ADP and GDP activate the enzyme, It is a reversible reaction that could function in:
- Amino acid catabolism as it transmits nitrogen from glutamate to urea in the urea cycle.
- Amino acid anabolism as it catalyzes amination of a-ketoglutarate by free ammonia to form glutamate.
III- Non-oxidative deamination (direct deamination)
The α-amino group of serine and threonine (amino acids containing the hydroxyl group) can be directly converted to NH3 without the removal of hydrogen (without oxidation). This reaction is catalyzed by serine and threonine dehydratase enzymes which need pyriodoxal phosphate as a coenzyme.
IV. Transdeamination
It is a transamination followed by oxidative deamination. The net conversion of the α-amino group to ammonia requires the action of glutamate transaminase and L-glutamate dehydrogenase.
You can download Science online application on Google Play from this link: Science online Apps on Google Play
Protein Classification, Globular & Fibrous protein, Simple, Compound & Derived proteins
Protein definition, structure, order, denaturation & Bonds responsible for protein structure
Protein structure, Classification, properties of amino acids & Formation of peptide bonds
Protein biosynthesis steps, site, importance, inhibitors & Protein maturation
Genetic code, formation of amino acid code & Steps of Protein synthesis