Substitution reactions, Chemical activity series, types of simple and double substitution reactions
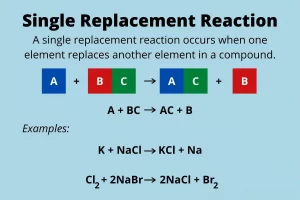
Substitution reactions occur when there is an active element replaces (substitutes) another less active element in another compound. Metals are arranged according to the degree of their chemical activity in a series, which is known as the “chemical activity series“.
Substitution reactions
Chemical activity series (C.A.S.) is the arrangement of metals in a descending order according to the degree of their chemical activity. The arrangement of metallic elements in the chemical activity series to compare between elements concerning the degree of chemical activity, where the more active element replaces the less active one. Substitution reactions are classified into two types:
Simple substitution reactions
Simple substitution reactions are chemical reactions in which one of the elements substitutes another less active element in a solution of one of its compounds.
Types of simple substitution reactions
- A metal substitutes the hydrogen of water.
- A metal substitutes the hydrogen of diluted acid.
- A metal substitutes another metal in its salt solution.
A metal substitutes the hydrogen of water
Metals that precede hydrogen in C.A.S. substitute hydrogen of water forming metal hydroxide and hydrogen gas evolves.
Substitution of sodium to hydrogen of water
Procedures
- Put a small piece of sodium by using tongs in a glass of water.
- Touch the glass of water carefully after ending the reaction.
Be careful when performing this reaction as this will lead to explosion and also burning. So, you must use a small piece of sodium (which is preserved under the surface of kerosene).
Observations
- Sodium burns with a pop sound (explosion).
- The glass of water becomes warm.
Conclusion:
Sodium substitutes the hydrogen of water giving sodium hydroxide and hydrogen gas evolves which burns with a pop sound and the reaction is accompanied by releasing of heat.
2Na + 2H2O → 2NaOH + H2 ↑ + Heat
A metal substitutes the hydrogen of diluted acid
Metals that precede hydrogen in C.A.S. substitute hydrogen of diluted acid-forming salt of the acid and hydrogen gas evolves.
Active metal + An acid → Salt of the acid + Hydrogen gas
Metals that come after hydrogen in the chemical activity series cannot substitute hydrogen of diluted acid (except under certain conditions).
Substitution of some metals to hydrogen of diluted acid
Procedures:
- Put equal amounts of dil. hydrochloric acid in three test tubes.
- Add 3 sheets of equal sizes as follows:
- A sheet of zinc in the first tube.
- A sheet of aluminium in the second tube.
- A sheet of copper in the third tube.
Observations:
Gas bubbles evolve immediately on adding the sheet of zinc. after a short time on adding the sheet of aluminium. No gas bubbles evolve on adding the sheet of copper.
Conclusion:
Zinc and aluminium replace the hydrogen of diluted acid forming a salt of the acid and hydrogen gas evolves because zinc and aluminium precede hydrogen in C.A.S. Copper doesn’t replace the hydrogen of diluted acid because copper comes after hydrogen in C.A.S.
Zn + 2HCl → ZnCl2 + H2 ↑
2Al + 6 HCl → 2AlCl3 + 3 H2 ↑
Cu + HCl→ No reaction
You can detect the evolution of hydrogen gas as a result of the substitution of metals to the hydrogen of diluted acids by approaching a burning match to it, so it burns with a pop sound.
Although aluminium comes before zinc in C.A.S., aluminium delays after zinc in the reaction with diluted hydrochloric acid due to the presence of a layer of aluminium oxide (Al2O3) on aluminium surface, which takes time to separate from aluminium, that delays the starting of occurrence of the reaction.
Both aluminium and zinc react with diluted hydrochloric acid, while copper doesn’t react with the same acid because both aluminium and zinc come before hydrogen in the chemical activity series, so they replace the hydrogen of acid, while copper comes after hydrogen in the chemical activity series, so it can’t replace the hydrogen of acid.
Sodium reacts with dil. HCI, while copper does not react with the same acid because sodium can replace the hydrogen of diluted acid as it comes before hydrogen in the chemical activity series, while copper comes after the hydrogen. Some metals replace other metals (in their salt solutions) that follow them in the chemical activity series.
(Metal A) + (Metal B salt solution) → Metal A salt solution + Metal B ↓
Substitution of magnesium to copper in copper sulphate solution
Procedures: Put a magnesium sheet in a test tube containing blue copper sulphate solution.
Observation: The blue colour of copper sulphate disappears and a red precipitate is formed.
Conclusion: Magnesium is more active than copper (as it comes before copper in the chemical activity series) so, it substitutes copper in copper sulphate solution forming magnesium sulphate (colourless) and copper precipitates (red ppt.)
Mg + CuSO4 → MgSO4 + Cu ↓
Magnesium can replace copper in its salt solutions, while the opposite doesn’t occur because magnesium precedes copper in C.A.S., so it replaces copper, but copper follows magnesium, so it cannot replace magnesium.
A red precipitate is formed when magnesium is added to copper sulphate solution because magnesium comes before copper in the chemical activity series, so it replaces copper in copper sulphate solution and copper precipitates as a red ppt.
The blue colour of copper sulphate disappears on putting a piece of zinc in it because zinc comes before copper in the chemical activity series, so it substitutes copper in copper sulphate solution forming colourless zinc sulphate.
Don’t keep silver nitrate solution in aluminium containers because aluminium precedes silver in C.A.S., so it replaces silver in silver nitrate solution which leads to eroding of aluminium containers. As the distance between metals increases in C.A.S., as the substitution becomes faster.
Application
Potassium (K) reacts instantly with water, while magnesium (Mg) reacts very slowly with cold water because the distance between K & H is greater than the distance between Mg & H in C.A.S (K is more active than Mg).
Double substitution reactions
Double substitution reactions are chemical reactions in which double substitution (exchange) occurs between the ions (radicals) of two different compounds to give two other new compounds.
Types of double substitution reactions
- The reaction between an acid and an alkali (neutralization reaction).
- Reaction between an acid and a salt.
- Reaction between two salt solutions
Reaction between an acid and an alkali (neutralization reaction)
A neutralization reaction is a reaction between an acid and alkali to form salt and water.
Acid + Alkali → Salt + Water
Example: Reaction between hydrochloric acid and sodium hydroxide to form sodium chloride (salt) and water.
NaOH + HCl → NaCl + H2O
On heating the (resultants) of the previous reaction, H₂O evaporates and NaCl (table salt) remains.
Reaction between an acid and a salt
Acids react with salts and the resultant depends on the type of both the acid and salt.
The reaction between diluted hydrochloric acid and sodium carbonate
Procedures:
- Put an amount of diluted hydrochloric acid in a flask.
- Put a little amount of sodium carbonate salt in a balloon.
- Insert the top of the balloon over the edge of the flask.
- Slowly turn over the balloon in a way that makes the amount of sodium carbonate fall into the flask.
Observation:
An effervescence happens inside the flask which makes the balloon is blown.
5. Carefully close the balloon and take it away of the flask.
6. Pass the gas formed inside the balloon into a clear limewater in a test tube for a short time.
Observation: The clear limewater becomes turbid.
Conclusion: Diluted hydrochloric acid reacts with sodium carbonate salt-forming sodium chloride, water, and carbon dioxide gas evolves which turbids the clear limewater.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2↑
Reaction between two salt solutions
The reaction between two salt solutions is accompanied by the formation of a precipitate (ppt.) (salt doesn’t dissolve in water].
Example:
The reaction between sodium chloride solution and silver nitrate solution: Sodium chloride solution reacts with silver nitrate solution to give sodium nitrate solution and a white precipitate of silver chloride.
NaCl + AgNO3 → NaNO3 + AgCl ↓
A white precipitate is formed by adding silver nitrate solution to sodium chloride solution due to the formation of silver chloride salt which doesn’t dissolve in water.
Oxidation and reduction reactions according to Traditional concept and Electronic concept
Chemical reactions, Types of thermal decomposition reactions and Air bags importance
Simple and double substitution reactions, Reaction between an acid & a salt
Chemical activity series, Chemical properties of metals & nonmetals
Types, importance, negative effects of chemical reactions, Sulphur & Carbon oxides harms