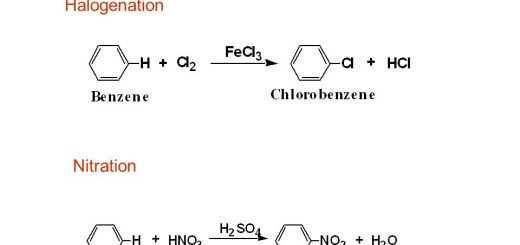
What is Valency?, Valencies of some metallic, nonmetallic elements and atomic groups
The atoms of noble elements are the most stable atoms due to the completeness of their outermost energy level with electrons, the atoms of other elements tend to enter in chemical reactions to reach the stable state to become their outermost energy levels completed with electrons by losing the outermost electrons as in metals, by gaining or sharing with electrons such as in nonmetals, this number of electrons is known as valency.
What is valency?
Valency is the number of electrons that an atom gains, loses or even shares during a chemical reaction, The valency of noble gases is zero because their outermost energy level is completely filled with electrons [ have 8 electrons except (He) has 2 electrons], The valency of an element is determined according to the number of electrons in the outermost energy level of its atom.
- Sodium (11Na) is monovalent (1) because it loses one electron during the chemical reaction.
- Chlorine (17Cl) is monovalent (1) because it gains or shares with one electron during the chemical reaction.
- Oxygen (8O) is divalent (2) because it gains or shares with two electrons during the chemical reaction.
- Magnesium (12Mg) is divalent (2) because it loses two electrons during the chemical reaction.
- Aluminium (13Al) is trivalent (3) because it loses three electrons during the chemical reaction.
- Argon (18Ar) is zero because it doesn’t lose, gain or share with any electrons due to the completeness of their outermost energy levels with electrons.
The valencies of some metallic elements
- Lithium (Li), potassium(K), sodium (Na) & silver (Ag) are monovalent (1).
- Calcium (Ca), Magnesium (Mg), lead (Pb), mercury (Hg) and zinc (Zn) are divalent (2).
- Aluminium (Al) and gold (AU) are trivalent (3).
There are some metallic elements have more than one valency:
- Copper (Cu): Copper Ι (monovalent) (1) & Copper IΙ (divalent) (2).
- Iron (Fe): Iron IΙ (Ferrous Fe+2) Divalent (2) & Iron IIΙ (Ferric Fe+3) trivalent (3).
The valencies of some nonmetallic elements
- Hydrogen (H), Chlorine (Cl), Fluorine (F), Bromine (Br) & Iodine (I) are monovalent (1).
- Oxygen (O) is divalent.
- Carbon (C) is tetravalent.
Some nonmetallic elements which have more than one valency such as:
- Sulphur (S) is divalent (2), tetravalent (4) and Hexavalent (6).
- Nitrogen (N) is trivalent (3) and pentavlent (5).
- Phosphorus (P) is trivalent (3) and pentavalent (5).
The atomic group
The atomic group (Radical) is a set of atoms of different elements joined together and behave like one atom during a chemical reaction, having its own valency and doesn’t exist solely (individually), the valency of an atomic group equals the number of charges which it carries.
Example: Bicarbonate group (HCO3)−, Its valency is monovalent, It consists of 5 atoms of 3 elements, One atom of hydrogen element (H), One atom of carbon element (C), three atoms of oxygen element (O).
The valencies of some atomic groups
- Hydroxide (OH−), Bicarbonate (HCO3)−, Nitrate (NO3)−, Nitrite (NO2)−, Ammonium (NH4)+ are monovalent (1).
- Carbonate (CO3)−2, Sulphate (SO4)−2 are divalent (2).
- Phosphate (PO4)−3 is trivalent (3).
Chemical formula
Compound molecules are formed as a result of a combination of atoms of different elements together. We can express a molecule of a chemical compound via a certain formula known as a chemical formula. the chemical formula is a formula that represents the number and the type of the atoms in a molecule.
The water molecule (H2O) consists of two atoms of hydrogen elements (H) and one atom of oxygen element (O), Sodium chloride molecule (NaCl) consists of one atom of sodium element (Na) and one atom of chlorine element (Cl).
How can you write a chemical formula for a compound?
- Write the name of the compound in words.
- Write the symbol of each element or atomic group down to its name.
- Write the valency down to each symbol or atomic group and exchange the valencies.
- Simplify the valencies (shortened as much as possible), you don’t have to write the one (1), in case of atomic groups if the number is not (1), Put the atomic group between brackets and write the number right down to it.
The formula for a compound starts from the left with a symbol of metal or hydrogen or a positive atomic group, it ends on the right with a symbol of a nonmetal or a negative atomic group, The word oxide means the combination of the metallic element or nonmetallic element with oxygen element.
An oxygen atom joins two atoms of sodium when composing one molecule of sodium oxide (Na2O) because oxygen is divalent, while sodium is monovalent, The chemical formula of sodium carbonate is (Na2CO3) because sodium is monovalent, while carbonate is a divalent group.
Potassium (19K) is monovalent, while oxygen (8O) is divalent because, during chemical reactions, potassium atom loses one electron, while oxygen gains or shares with two electrons to complete their outermost shell. Magnesium (12Mg) is divalent, while aluminium (13Al) is trivalent because, during chemical reactions, magnesium atom loses two electrons, while aluminium atom loses three electrons.
Both sodium (11Na) and chlorine (17Cl) are monovalent although they have different atomic numbers because, during chemical reactions, sodium atom loses one electron, while chlorine atom gains or shares with one electron to complete their outermost shell.
The valency of noble gases is zero because their outermost energy levels are completely filled with electrons, so, they don’t lose, gain or share with any electrons. An oxygen atom joins two atoms of sodium when composing one molecule of sodium oxide because oxygen is divalent, while sodium is monovalent.
Both nitrate & carbonate groups have the same number of atoms but differ in their valencies because nitrate group (NO3)− consists of four atoms and it is a monovalent group, while carbonate group (CO3)−2 consists of four atoms but it is a divalent group. Both nitrite & nitrate groups differ in the number of atoms and having the same valency because both are monovalent but nitrate (NO3)− group consists of 4 atoms, while nitrite (NO2)− group consists of 3 atoms.
The chemical formula of sodium carbonate is (Na2CO3) because sodium is monovalent, while carbonate is a divalent group so, two atoms of sodium combine with one atom of carbonate group. The chemical formula of water is (H2O) because oxygen is divalent, while hydrogen is monovalent, so two atoms of hydrogen combine with one atom of oxygen.
Atoms components, Rutherford and Bohr’s Atomic Models
How can you calculate the valency of each element?
Molecular orbital theory & Valence shell electron pair repulsion (VSEPR) theory
Chemical bonds, Ionic bonds, Properties & types of covalent bonds
Types of compounds, Properties of Acids, Bases (alkalis), Oxides and Salts