What are the properties of nitrogen gas?
Nitrogen gas (N2) is mainly found in the atmosphere; it is also found in the Earth’s crust in the form of nitrates, it is found in the organic form in living or dead plants, and in the organisms that form the humus.
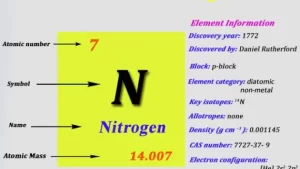
Nitrogen gas
Nitrogen gas (N₂) is a colorless, odorless, tasteless, and inert gas that makes up about 78% of the Earth’s atmosphere by volume. It’s a vital element in both industrial processes and living organisms.
- Chemical Formula is N₂.
- Molar Mass is 28.02 g/mol.
- State at Room Temp is Gas.
- Color/Odor: Colorless and odorless.
- Reactivity: Very low under normal conditions.
- Density (at STP): 1.2506 g/L.
Natural Role of Nitrogen
- In the Atmosphere: A Major component of air.
- In Living Organisms: Found in amino acids, proteins, DNA, and RNA.
- Nitrogen Cycle: Nitrogen is fixed from the atmosphere by certain bacteria and converted into compounds that plants and animals can use.
Industrial & Practical Uses
- Inert Atmospheres: Nitrogen is used to prevent oxidation in processes like welding or food packaging.
- Cryogenics: Liquid nitrogen can be used for freezing and transporting biological samples.
- Chemical Manufacturing: Nitrogen is used in the production of ammonia (Haber process).
- Electronics: Nitrogen protects sensitive components during manufacturing.
- Tire Inflation: Nitrogen is used in aircraft and racing cars for more stable tire pressure.
Safety Considerations
- In high concentrations, nitrogen can displace oxygen, posing a suffocation risk in confined spaces.
- It’s not poisonous, but it can be dangerous when it displaces breathable air.
Nitrogen gas properties
Nitrogen gas is a colorless, odorless, and tasteless gas that makes up 78 % of the atmosphere volume, it does not help in burning, it is non-flammable, and it will not support combustion.
Nitrogen gas scarcely dissolves in the water, it is slightly lighter than air and slightly soluble in water, and it condenses at its boiling point -195.8o C (-320.4o F) to a colorless liquid that is lighter than the water.
Nitrogen compounds are formed naturally through the biological activity, they are also formed at a high temperature or at a moderate temperature with the aid of catalysts, it will combine with active metals such as lithium, magnesium, and titanium to form nitrides at high temperatures.
Nitrogen gas is necessary for many biological processes and is used as a fertilizer in the form of ammonia or ammonia-based compounds, and the compounds formed with halogens and certain organic compounds can be explosive.
Nitrogen gas is not an inert gas, it forms nitric oxide and nitrogen dioxide with oxygen, ammonia with hydrogen, and nitrogen sulfide with sulfur.
Nitrogen gas is used in industries such as petroleum processing, chemicals, pharmaceuticals, glass, and fabrication processes, steelmaking, and other metals refining, pulp, paper manufacture, and it is used in healthcare.
Nitrogen gas may be referred to as LIN or LN in its liquid form, and GAN or GN in its gaseous form. Gaseous nitrogen is valued for inertness, and it is used to shield potentially reactive materials from contact with oxygen.
Nitrogen gas does not easily react with a lot of elements as it is an inactive element; it has a neutral effect on litmus paper, and it can be condensed into a liquid state.
When liquid nitrogen is vaporized and warmed to ambient temperature, it absorbs a large quantity of heat, and the combination of inertness and its intensely cold initial state makes liquid nitrogen an ideal coolant for certain applications, such as food freezing.
Liquid nitrogen is used to cool the materials that are heat sensitive or normally soft to allow machining or fracturing, such as tires, plastics, some metals, and pharmaceuticals, so liquid nitrogen is valued for coldness as well as inertness.
Nitrogen gas combines with the lighted magnesium ribbon, forming a white substance that reacts with the water, forming ammonia gas, which has a pungent smell.
Nitrogen gas is produced in large volumes in both gas and liquid form by cryogenic distillation, and cryogenic processes can produce very pure nitrogen.
You can subscribe to Science Online on YouTube from this link: Science Online
Elements of p-block, General properties of group 5A elements (group 15)