Ammonia metabolism and properties, Five enzymes of urea cycle and Amino Acid Metabolism Glycine
Ammonia is known as anhydrous ammonia, It is produced in the human body and is commonly found in nature, It is essential in the body as a building block for making proteins and other complex molecules, In nature, ammonia occurs in soil from bacterial processes, It is produced when plants, animals and animal wastes decay.
Properties of ammonia
It is a colorless highly irritating gas with a sharp suffocating odor, It dissolves easily in water to form an ammonium hydroxide solution which can cause irritation and burns, Ammonia gas is easily compressed and forms a clear, colorless liquid under pressure, It is shipped as a compressed liquid in steel cylinders, Ammonia is not highly flammable, but containers of ammonia may explode when exposed to high heat.
Sources of blood ammonia
- From amino acids: The liver mainly and many tissues form NH3 from amino acids by combined transaminases and glutamate dehydrogenase, a.a oxidases, and non-oxidative deamination.
- From glutamine by: Renal glutaminase: The kidney produces ammonia, most of it is excreted as NH4+ which is an important mechanism for maintaining the body’s acid-base balance. Intestinal glutaminase: Intestinal mucosal cells obtain glutamine from the blood or from the digestion of dietary protein.
- From amines: whether dietary amine or monoamine hormones by amine oxidase.
- From catabolism of purines and pyrimidines.
- From bacterial action in the intestine either from dietary protein residue or from urea diffuses into the intestine and is hydrolysed by the urease of intestinal bacteria (This is of significance in cases of kidney failure)).
Fates of ammonia (Removal of ammonia)
- Amination of α-ketoacid to form non-essential amino acids and other biosynthetic reactions.
- Glutamine synthesis in the brain, liver, muscle, and renal tissues (4%).
- The majority of NHcytoplasm (90%) will produce urea in the liver by the urea cycle.
- Excretion in urine up to 1gm/24 hours urine.
- Traces in the blood (up to 100 ug/dl).
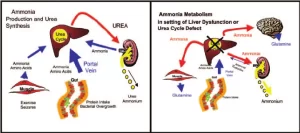
Metabolism of ammonia
In peripheral blood (Systemic blood) ammonia level is less than 100 µg/dl. (Ammoniacal encephalopathy) is defined as the toxicity of the brain due to an increase in the NH3 level in the systemic blood. This increased ammonia will be fixed to α ketoglutaric acid to form glutamic acid then glutamine leading to interference with the citric acid cycle so decrease ATP production in the brain cells.
Causes:
- Congenital: The 5 types of hyperammonaemia due to enzyme deficiencies in the urea cycle.
- Acquired:
- Liver disease as cirrhosis due to failure of urea formation and glutamine synthesis.
- Portocaval shunt as in bilharziasis. Normally, ammonia, which is formed by the action of the bacterial flora on amino acids, residue in the intestine, is removed by the liver. But in the case of a portocaval shunt, the portal blood bypasses the liver detoxication of toxic ammonia to the systemic circulation to the brain.
- Gastrointestinal bleeding by the action of the bacterial flora on the blood urea and thus NH3 is released in large amounts.
Manifestations of ammonia intoxication
- Tremors
- Blurred vision
- Slurred speech
- Vomiting
- Confusion is followed by coma and death.
Krebs urea cycle (ornithine cycle for urea formation)
Urea is formed in the liver mainly some in the brain and renal tubules from one molecule of CO₂ and two molecules of NH3 using 3 ATP’s. It is released into the blood with a level of 20-40 mg/dl of serum. It is cleared by the kidney to the urine with a level of 20-40 g/24 hours urine collection.
It is the major end product of nitrogen catabolism in humans representing 80-90% of the nitrogen excreted. Five reactions each of them utilises a specific enzyme in the urea cycle. The first 2 reactions of the urea cycle are mitochondrial and the rest 3 reactions are cytoplasmic.
Five enzymes of the urea cycle
- Carbamoyl phosphate synthase.
- Ornithine transcarbamoylase (citrulline synthase).
- Argininosuccinate synthetase.
- Argininosuccinase.
- Arginase.
Link between krebs’ urea cycle and krebs’ tricarboxylic acid cycle:
- The fumarate resulting from reaction number 4 (in Krebs urea cycle), is an intermediate in the citric acid cycle. It could be converted in the cytoplasm to aspartate which is needed for the urea cycle.
- The CO2 used urea cycle comes mainly from Krebs’ tricarboxylic acid cycle.
- Aspartate in the urea cycle may be generated from oxaloacetate of the Citric acid cycle.
The first NH2 group comes from L-glutamic acid by L-glutamate dehydrogenase, The second NH2 group comes from the amino group of aspartic acid.
Regulation of urea cycle
- Carbamoyl phosphate synthase I is the rate-limiting enzyme of the urea cycle. It is active only in the presence of N-acetyl glutamate (an Allosteric activator).
- Excess ammonia formation stimulates urea formation.
- High arginine level stimulates N-acetyl glutamate synthase enzyme) thus increases urea formation.
- High urea level inhibits carbamoyl phosphate synthase (reaction 1), ornithine transcarbamoylase (reaction 2), and arginase enzymes (reaction 5).
Metabolic fate of the carbon skeleton of amino acids
The carbon skeleton after removal of the amino group is oxidized or converted to glucose (in case of glucogenic amino acids) or ketone bodies and fat (in case of ketogenic amino acids) or to both (in mixed amino acids). This occurs according to body needs.
- Ketogenic Amino Acids: only leucine
- Both Ketogenic and Glucogenic Amino Acids (mixed): Phenylalanine, Tyrosine, Tryptophan, Isoleucine, and Lysine.
- Glucogenic Amino Acids: All the remaining of the 20 naturally occurring amino acids.
Amino Acid Metabolism Glycine
It is a neutral, non-essential, and glucogenic amino acid.
I- Synthesis of glycine
- From serine by serine hydroxymethyl transferase.
- From CO₂ and NH3 by glycine synthase (glycine cleavage).
- From glyoxylate by transamination.
II- Catabolism of Glycine
- By glycine cleavage system (a reversible reaction to its synthesis by glycine synthase), it is the major pathway for glycine catabolism (also for serine catabolism) in humans.
- By amino acid oxidase (oxidative deamination). Glycine is catabolized to glycoxylic acid.
- By serine hydroxymethyl transferase to serine (a reversible reaction for its synthesis), then serine can be catabolized to pyruvate by serine dehydratase enzyme. This proves that both glycine and serine are glucogenic amino acids.
III- Functions and derivatives
- It is Glucogenic as it forms serine, which by serine dehydratase gives pyruvate.
- Glycine is a source of one carbon moiety as it is the source of one carbon present in methylene tetrahydrofolate and formyl tetrahydrofolate which are formed during glycine catabolism.
- Synthesis of serine through serine hydroxymethyl transferase enzyme.
- Synthesis of bile salts e.g sodium glycocholate.
- Synthesis of glutathione.
- Synthesis of heme (porphobilinogen).
- Synthesis of purines: Glycine is the source of C4, C,5 and N7 of the purine ring.
- Detoxification of aromatic acids: Benzoyl co A + glycine → Hippuric acid
- Synthesis of creatine and creatinine
IV- Metabolic inborn errors
Primary hyperoxaluria: it is a hereditary metabolic disease due to a deficiency of the enzymes that metabolize glyoxylate, which is oxidized to oxalate. Oxalate is excreted in urine as calcium oxalate with an increased risk of urinary stone formation.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download Science online application on Google Play from this link: Science online Apps on Google Play
Protein metabolism, Fate of absorbed amino acids and Biological importance of transamination
Protein Classification, Globular and Fibrous protein, Simple, Compound and Derived proteins
Protein definition, structure, order, denaturation and Bonds responsible for protein structure
Protein structure, Classification, properties of amino acids and Formation of peptide bonds
Protein biosynthesis steps, site, importance, inhibitors and Protein maturation