Evolution concept of the atomic structure, Atomic theory and Properties of cathode rays
A long time ago man was asking about the nature of matter and its structure, while trials are done by scientists to answer this question across different eras, the concept of the atomic structure has evolved, scientists were interested in studying the atomic structure such as Heisenberg, Pauli, Schrodinger, Bohr, De Broglie, Einstein and Planck.
Democritus’s (Greek philosopher) idea
He imagined the possibility of dividing any piece of matter into smaller parts, then dividing those parts into smaller particles, and so on, until we reach an undividable fragment, he named it an atom, In the Greek language: A means no and tom means divide.
Aristotle’s idea (4th century B.C)
He rejected the concept of the atom and believed that all matter – whatever its nature – is composed of four components, which are water, air, dust, and fire.
It was believed that cheap metals such as iron or copper could be changed into precious ones such as gold by changing the ratios of these four constituents.
This miserable idea caused a retard of development in chemistry science for more than a thousand years because the scientists were busy by changing cheap metals into precious ones.
Boyle’s idea (1661)
The Irish scientist Boyle refused Aristotle’s idea about the name of the substance and gave the first definition of the element, The element is a pure simple substance that can not be changed to simpler forms by traditional chemical methods.
Dalton’s model of the atom (1803)
The English scientist John Dalton stated the first theory about the atomic structure.
The main postulates of Dalton’s atomic theory:
- The element is composed of very minute particles, which are named atoms.
- The atom is a very minute undividable solid particle.
- The atoms of the same element are similar in mass, but they are different from the atoms of any other element.
- The compounds are formed by the combination of atoms of different elements in simple numerical ratios.
Thomson’s model of the atom (1897)
The scientist Thomson carried out many experiments on the electric discharge through gases from which he discovered the cathode rays.
Discovery of cathode rays :
It was known that gases do not conduct electricity under normal conditions of pressure and temperature, However, gases conduct electricity in a discharge tube whose two poles are connected to an electric source of a suitable high potential difference and under a very low pressure.
If the potential difference between the two poles of the vacuum glass tube exceeds 10000 volts, a stream of invisible rays is emitted from the cathode (the negative pole), causing a fluorescent glow on hitting the tube wall, These rays were named by cathode rays.
Cathode rays: A stream of invisible rays was emitted from the cathode, causing a fluorescent glow hitting the discharge tube wall under very low pressure and a potential difference of about 10000 volts, It was later known that they are composed of minute particles named electrons.
Properties of cathode rays
- They consist of very fine negatively charged particles (electrons).
- They move in a straight line.
- They have a thermal effect.
- They are affected by both electric and magnetic fields.
- They do not differ either in behavior or in nature if the material of the cathode or the used gas is changed, this is a strong evidence that it is a fundamental constituent of any matter.
In light of the electrical discharge experiment, Thomson suggested a new atomic model for the atom.
The postulate of Thomson’s model:
He considered the atom as a solid sphere of uniform positive electric charges in which a number of negatively charged electrons is embedded to make the atom electrically neutral.
Rutherford’s model of the atom (1911)
Rutherford’s students Geiger and Marsden performed his famous laboratory experiment.
The used tools: A deep lead box containing a source of alpha particles ( α ) inside it, A metal sheet covered from the inside with a layer of zinc sulphide ZnS which is used to detect the alpha particles, because it glows at the site of collision with alpha particles and a very thin gold foil.
It was allowed for alpha particles to collide on a metal sheet, where it was possible to define the location and number of alpha particles by counting the glows that appeared on the metal sheet, A very thin gold foil was placed between the beam of alpha particles and the metal sheet.
The vast majority of α-particles penetrated the gold foil and hit the same places in which they appeared before placing the gold foil.
A very small percentage of α-particles did not penetrate the gold foil and reflected back, where some flashes appeared in front of the foil, Some of α-particles penetrated the foil but were deflected.
Most of the atomic volume is a space and the atom is not uniformly dense as proposed by Dalton and Thomson, The atom contains a tiny part of a very high density (was named the nucleus).
The dense part of the atom (where most of the mass is present) appears to have a similar positive charge to that of α-particles, based on his experiment and from the experiments of other scientists, Rutherford designed his atomic model as follows :
The postulates of Rutherford’s atomic theory:
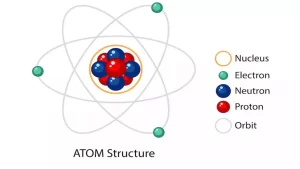
Atom is an extremely small-sized particle, It has a complicated structure that resembles the solar system, since it’s composed of a central nucleus (representing the Sun), where the electrons revolve around (representing the planets).
The nucleus is much smaller than the atom and most of the atomic mass is concentrated in it, There is a vast space between the nucleus and the orbits of electrons (the atom is not uniformly dense), It is positively charged.
Electrons have negligible mass compared to that of the nucleus, Their charge is negative and equals the nuclear positive charge (the atom is electrically neutral).
Electrons travel around the nucleus at a tremendous speed in special orbits, despite the mutual attraction between them and the nucleus, This attraction force is equal in quantity and opposite in direction to the centrifugal force resulting from the electron revolving around the nucleus.
Electrons do not fall into the nucleus in spite of the attraction force between them, because the attraction force equals the centrifugal force resulting from the revolving of electrons around the nucleus.
Rutherford’s theory failed to explain the atomic structure because it did not explain the system in which electrons revolve around the nucleus.
Atoms components, Rutherford and Bohr’s Atomic Models
Atomic emission spectra, Bohr’s atomic theory, Wave mechanical theory of the atom