Graduation of electronegativity of elements in modern periodic table
Electronegativity is the ability of the atom in the covalent molecule to attract the electrons of the chemical bond towards itself, There is an inverse relationship between the atomic size of the atom of an element, and its electronegativity in the modern periodic table.
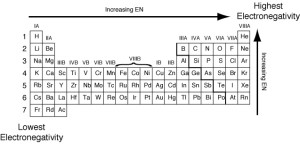
In periods in the modern periodic table. the electronegativity increases by increasing the atomic number (from left to right). The electronegativity for the elements of the same period is directly proportional to the atomic number.
In groups in the modern periodic table, the electronegativity decreases by increasing the atomic number (from up to down). The electronegativity for the elements of the same group is inversely proportional to the atomic number.
The inert gases have not electronegativity as they do not enter in the chemical reaction under normal conditions.
The difference in the electronegativity between the elements forming the compound molecules which determines the kind of bond between the elements if it is an ionic bond or a covalent bond.
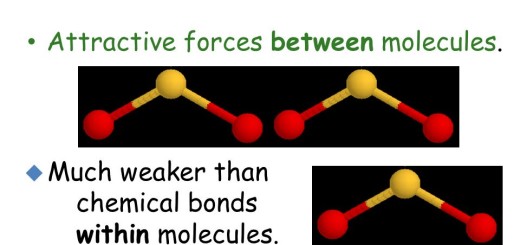
The difference in the electronegativity between the elements forming the covalent molecules which measures the polarity of these molecules, So, the covalent compounds are classified into polar compounds and non-polar compounds.
The polar compounds are the covalent compounds in which the difference in the electronegativity between their elements is relatively high.
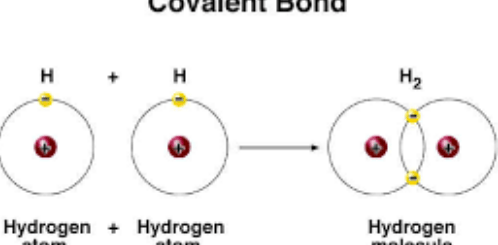
When the difference in the electronegativity between the elements of a covalent compound molecule equals zero, the bond in this molecule is called the pure covalent bond. As in oxygen molecule and hydrogen molecule.
You can download Science online application on google play from this link: Science online Apps on Google play
Graduation of the properties of the elements in the modern periodic table
The attempts of the elements’ classification
The description of the modern periodic table
Modern periodic table and classification of Elements
Chemical combination, Types of bonds (Chemical bonds & Physical bonds)