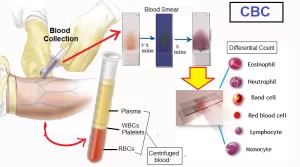
Blood analysis, Hemolysis importance, structure, types and hemolysis test results
Blood consists of plasma, red blood cells, white blood cells, and platelets, It has many different functions such as transporting oxygen and nutrients to the lungs and tissues, forming blood clots to prevent excess blood loss, carrying cells and antibodies that fight infection, and bringing waste products to the kidneys and liver, which filter and clean the blood, regulating body temperature.
Blood
Blood transports oxygen and nutrients to the cells and carries away carbon dioxide and other waste products, It is a transport liquid pumped by the heart to all parts of the body, after which it is returned to the heart to repeat the process.
Blood consists of:
- Fluid portion called plasma which occupies 55-66 percent of the volume of whole blood.
- Formed elements that are differentiated to
- Red blood corpuscles (erythrocytes).
- White blood cells (leucocytes),
- Blood platelets. (thrombocytes).
If blood is taken out from an artery or a vein it undergoes clotting within a few minutes. It changes into a gelatinous mass, which on standing squeezes out a clear straw-coloured fluid called the serum.
On the other hand, if a little of sodium or potassium oxalate is added to the blood just after its withdrawal from a blood vessel, blood will be prevented from clotting, and on standing or by centrifugation all the corpuscles will settle down, the clear supernatant fluid in this case is called plasma.
Therefore, the difference between plasma and serum lies in the presence of the clotting material, fibrinogen, still present in the solution of plasma.
Plasma = whole blood − formed elements
Serum = plasma − clotting material
Red Blood Corpuscles
Erythrocytes are non-nucleated cells composed of 60-65% water and 35-40% solids. The greater part of these solids is the metallo-chromoprotein, haemoglobin. Each cell consists of a special membrane, having the appearance of a meshwork, and is called the stroma. The membrane surrounds a concentrated solution of haemoglobin. The amount of haemoglobin in whole blood varies from 13-19 g%.
The stroma is composed of a mixture of proteins and lipids such as lecithin, cephalin, and cholesterol, and it acts as a semi-permeable membrane with great selective properties. The red cells possess an osmotic pressure which is equivalent to that of the plasma surrounding them.
The osmotic pressure is again equivalent to that of 0.9% NaCl solution. A solution of NaCl with this concentration is said to be isotonic and is usually described as physiological saline. Blood analysis may be carried out on whole blood, plasma, or serum depending distribution of the substance which is being studied.
There are a few general principles to be considered in the choice of sample to be analyzed:
- If the substance to be analyzed is present in both red cells and plasma at the same concentration, either serum (or plasma), or blood may be used.
- In some determinations, it is necessary to use serum or plasma, since clinically significant changes in serum in disease states, are much greater than that of erythrocytes. In other determinations, it is important to use red blood cells in order to obtain reliable.
Preparation of the samples
- Whole blood: When the analysis is to be performed on whole blood, the blood must be firstly diluted with a suitable diluent, e.g. water. This process results in haemolysis of erythrocytes. As in the case of haemoglobin, lactate, pyruvate, and ammonia.
- Serum: The blood will coagulate in the tube if left for some time (a few minutes). Then separation of the serum will be done by centrifugation. As in the case of glucose, cholesterol, albumin, and enzymes.
- Plasma: The only difference between serum and plasma is the presence of clotting factors (mainly fibrinogen) in the latter. So whenever plasma is to be employed in clinical determinations, an anticoagulant must be promptly & immediately mixed with blood sample just after its collection to prevent blood coagulation (as in the case of testing for e.g. fibrinogen, prothrombin and others).
Anticoagulants
The most common anticoagulants used in biochemical laboratories are:
- Potassium oxalate: It reacts with Ca++ and yields insoluble calcium oxalate, and inhibits its participation in the coagulation mechanism.
- Sodium fluoride: It prevents the participation of Ca++ in the blood coagulation mechanism by the formation of insoluble calcium fluoride.
- Citrate: Sodium or potassium citrate may be used as an anticoagulant. It inhibits blood coagulation by combining with Ca++ to form calcium citrate that is soluble but not ionizable.
- EDTA: (di-Sodium salt of ethylendiamine tetra-acetic acid): It inhibits blood coagulation forming a soluble salt of calcium that is undissociable preventing the liberation of calcium as Ca++ and the participation of this cation in blood coagulation.
- Heparin: It inhibits the action of various enzymes involved in blood coagulation.
Take care of:
Effect of fist clenching
Contraction of forearm muscles causes release of potassium → Can cause 1-2 mmol/L increase (Intracellular electronegativity declines during depolarization muscle cells favoring release of potassium rather than its uptake).
Effect of tourniquet
- Greater hemoconcentration: 3 min vs. 1 min.
- Venous stasis leads to increased anaerobic glycolysis, accumulation of lactate, and low pH.
- The hypoxic effect leads to leakage of K from cells: causing a spurious increase in serum K.
- A drop in blood pH results in the release of ionic Ca & Mg bound to albumin.
- Drop in blood pH: increase in free drug level.
Sample taken from drip arm while drip running
If it is a dextrose saline drip, it will falsely increase glucose, and decrease sodium, chloride, urea, and creatinine.
After centrifugation: does the specimen show haemolysis, icterus, or lipaemia?
Substances with higher concentrations inside the red cells will leak into the serum
- Higher concentration in red cells than in serum: K, phosphate, AST, LDH.
- The lower concentration in red cells than in serum: Na, Cl, and carbon dioxide.
- Concentration about equal in red cells and serum: creatinine, glucose, urea, and urate.
- Bilirubin is known to interfere with measurements of creatinine, Cholesterol, Glucose, TG, and Uric acid.
Errors in the laboratory
As they are part of the overall healthcare system, clinical laboratories are prone to medical errors. While the total testing process is typically divided into three main phases (pre-, intra-, and post-analytical), a body of evidence demonstrates that, currently, the pre-and post-analytical steps of the laboratory testing process are more error-prone than the analytical steps.
Laboratory errors may be defined as “any defect from ordering tests to reporting results and appropriately interpreting and reacting on these results”. In recent decades, standardization, automation, and technological advances have significantly improved the analytical reliability of laboratory results and decreased error rates.
Laboratory errors may be :
- Preanalytical → Patient & specimen variation.
- Analytical → Performance of the test.
- Postanalytical→ Reporting, Recording, and Interpreting results
Blood Hemolysis
Any change in the osmotic pressure of the medium surrounding the red cells will be reflected in their size. In a hypotonic solution, the corpuscles will increase in size due to the passage of water from the outer medium to the inside until the membrane can not resist the distention and ruptures.
On the other hand, if the red cells are placed in a hypertonic solution the reverse happens, water passes out from the cells to the outer medium and this results in the diminution of their size and they become crenated. Rupturing of RBCs and passage of haemoglobin to the outer median is known as haemolysis.
You can subscribe to Science Online on YouTube from this link: Science Online
You can download the Science Online application on Google Play from this link: Science Online Apps on Google Play
Cloning of DNA sequences, Importance of recombinant DNA & Uses of human genome
Molecular technology (Genetic Engineering) advantages, Techniques and uses
Importance of Nucleosides, Nucleotides, Purines, Pyrimidines & Sugars of nucleic acids
Regulation of the cell cycle, DNA synthesis phase, Interphase & Mitosis